Session Information
Session Type: Poster Session D
Session Time: 1:00PM-3:00PM
Background/Purpose: Guselkumab (GUS) is a human monoclonal antibody targeting the IL-23 p19 subunit. In the Phase 3b COSMOS trial (NCT03796858), GUS significantly improved disease signs/symptoms vs placebo (PBO) in patients (pts) with psoriatic arthritis (PsA) and an inadequate response (IR) to TNF inhibitor (TNFi) therapy. The primary endpoint of American College of Rheumatology (ACR)20 response at Week (W)24 was achieved, with the benefit of GUS vs PBO consistent across subgroups.1 In this post-hoc analysis, we evaluated if response to GUS across disease domains was maintained through 1 year across various subgroups of these TNFi-IR PsA pts.
Methods: In COSMOS, adults with active PsA (swollen joint count [SJC] ≥ 3 and tender joint count [TJC] ≥ 3) with a lack of efficacy/intolerance to 1–2 TNFi therapies were randomized (2:1) to GUS 100 mg or PBO. Pts in the GUS group were treated at W0, W4, then every 8 weeks (Q8W) to W44; those in the PBO group were treated at W0, W4, W12, and W20, with planned crossover to GUS 100 mg at W24 followed by GUS at W28, W36, and W44. Pts with < 5% improvement from baseline (BL) in SJC and TJC qualified for W16 early escape (EE); EE pts receiving GUS continued treatment, while pts receiving PBO crossed over to GUS. In this analysis, efficacy of GUS in subgroups was evaluated at W24 and W48 via joint (ACR20/50 response), skin (Psoriasis Area and Severity Index [PASI]100 response), and multi-domain (minimal disease activity [MDA] response) outcome measures. Subgroups were defined by selected demographics, disease characteristics, and ongoing medications at BL: sex, body mass index (BMI), SJC, TJC, PsA duration, % psoriatic body surface area (BSA), and conventional synthetic disease-modifying antirheumatic drug (csDMARD) use. Odd ratios (ORs) and 95% confidence intervals (CIs) based on Fisher’s exact test for GUS vs PBO are shown overall and for each subgroup at W24. No treatment comparison was performed after W24. Pts who discontinued and/or correctly met EE criteria before W24 were imputed as nonresponders. Missing data were also imputed with no response through W48.
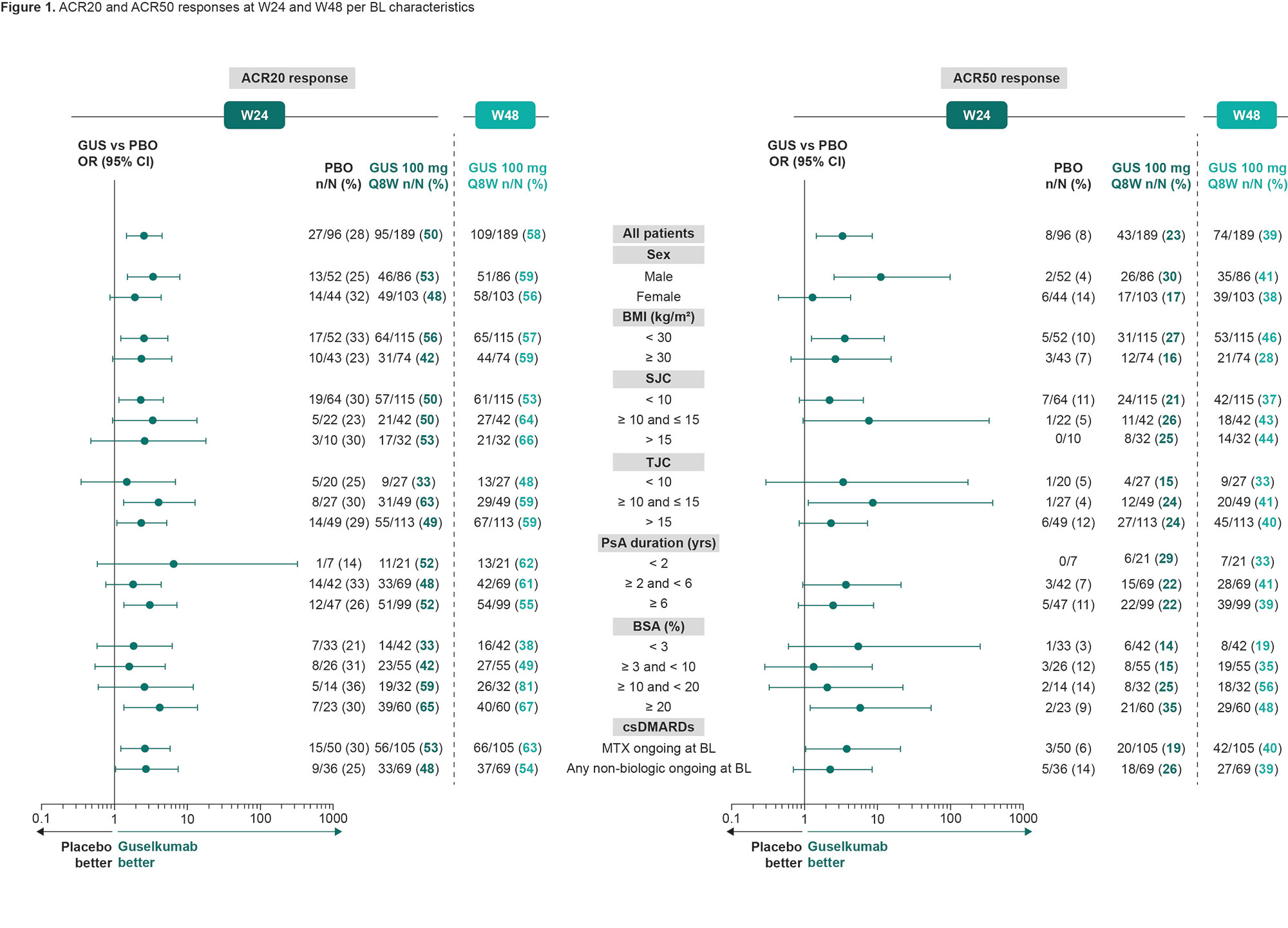
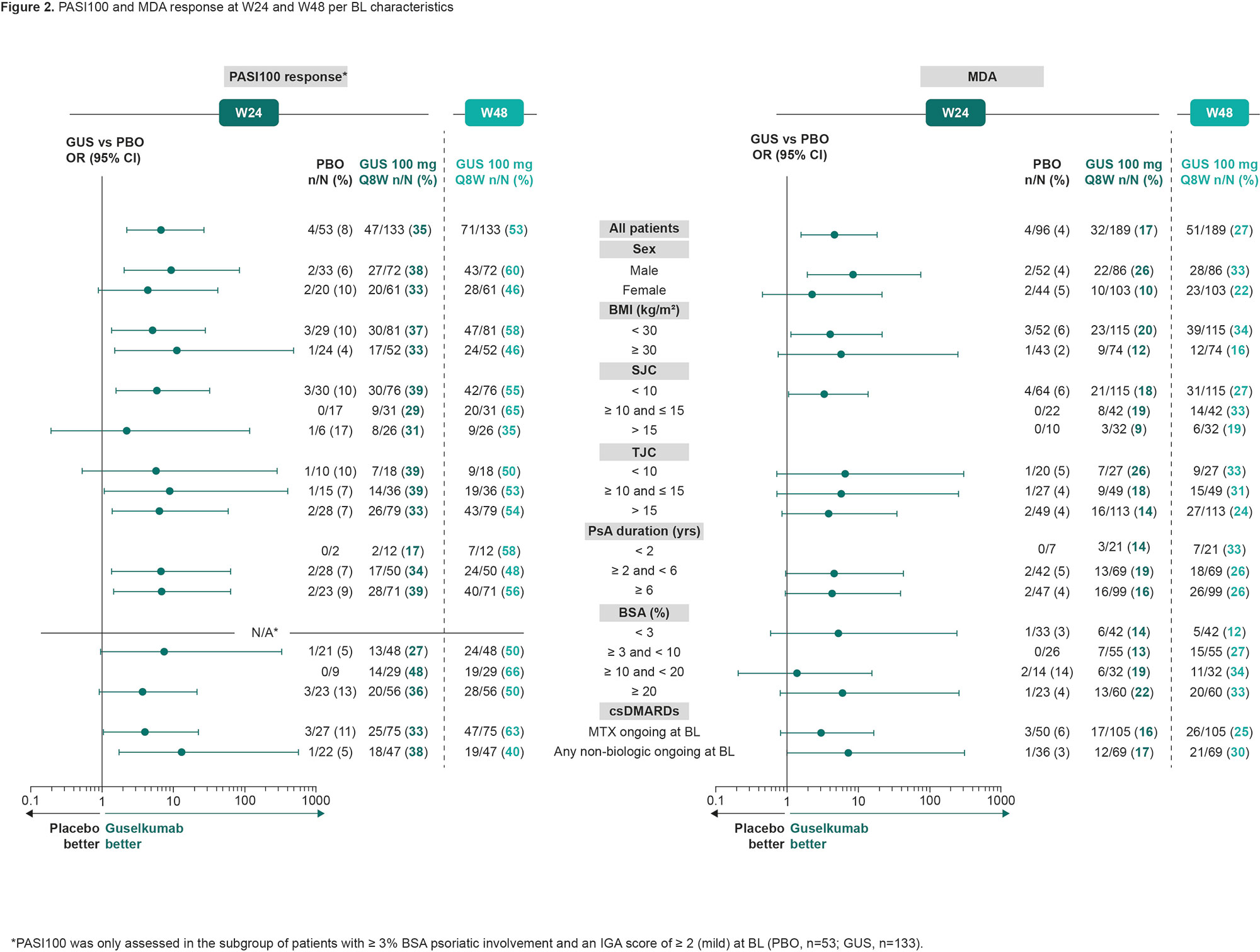
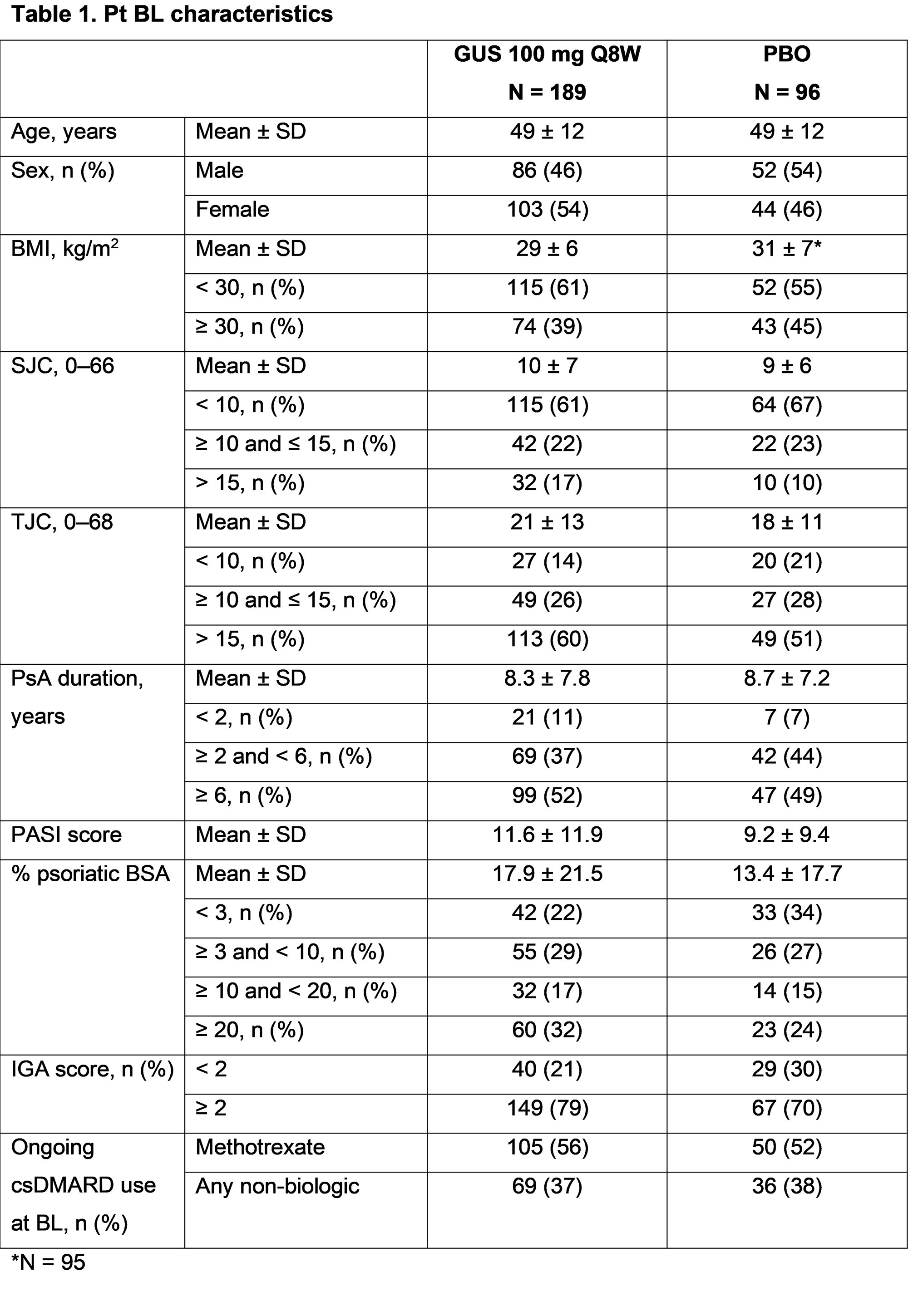
Results: Overall, 285 pts were randomized to GUS (n = 189) or PBO (n = 96). BL characteristics were generally similar between treatment groups, although numeric differences were observed for the proportion of females, joint symptoms, and skin involvement in GUS vs PBO pts (Table 1). At W16, 39 (21%) pts in the GUS group and 45 (47%) pts in the PBO group were assigned to EE. In this analysis, joint, skin, and multi-domain response rates at W24 were numerically greater in GUS vs PBO pts, with the benefit of GUS consistent across all subgroups of adequate sample size (Fig 1 [ACR20 and ACR50] and Fig 2 [PASI100 and MDA]). Response rates with GUS were maintained, or in most cases numerically increased, from W24 to W48, regardless of BL subgroup.
Conclusion: GUS 100 mg Q8W led to consistent improvements vs PBO in joint, skin, and multi-domain outcomes at W24 across subgroups of TNFi‑IR PsA pts defined by selected demographics, disease characteristics, and ongoing medications at BL. Response to GUS was maintained or further improved through 1 year of treatment regardless of BL subgroup.
1. Coates LC et al. Ann Rheum Dis 2022;81:359–69
To cite this abstract in AMA style:
McInnes I, Sewerin P, Sharaf M, Efficace M, Shawi M, Perate M, Zimmermann M, Coates L. Sustained Response to Guselkumab Regardless of Baseline Demographic, Disease, and Medication Characteristics in Patients with Active Psoriatic Arthritis and an Inadequate Response to TNF Inhibitors: Results from a Phase 3b Trial [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/sustained-response-to-guselkumab-regardless-of-baseline-demographic-disease-and-medication-characteristics-in-patients-with-active-psoriatic-arthritis-and-an-inadequate-response-to-tnf-inhibitors-r/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/sustained-response-to-guselkumab-regardless-of-baseline-demographic-disease-and-medication-characteristics-in-patients-with-active-psoriatic-arthritis-and-an-inadequate-response-to-tnf-inhibitors-r/