Session Information
Date: Tuesday, November 9, 2021
Title: Abstracts: Spondyloarthritis Including PsA – Treatment II: Biologic Therapies (1943–1946)
Session Type: Abstract Session
Session Time: 4:15PM-4:30PM
Background/Purpose: Among treatment options for PsA, IL-23/12 inhibition with ustekinumab (UST) was the first new biologic after TNF inhibitors (TNFi). Few data compare long-term effectiveness between UST and TNFi. Here we present the final 3-year analysis from the real-world PsABio study, focusing on the achievement of minimal disease activity (MDA)/very low disease activity (VLDA) and low disease activity (LDA) or remission, as measured by clinical disease activity in psoriatic arthritis (cDAPSA).
Methods: The PsABio study (NCT02627768) evaluated effectiveness, tolerability and persistence of 1st, 2nd, or 3rd-line UST or TNFi in patients (pts) with PsA. Here we present proportion of pts reaching MDA/VLDA and cDAPSA LDA or remission up to 3 years (assessments for pts under the initial treatment at 3 years). Descriptive statistics included the last observation carried forward (LOCF) endpoint created in case of missing 3-year effectiveness data; for example, due to COVID-19. Cohort comparison was done among pts who stayed on their initial UST or TNFi treatment for the full 3 years (remainer analysis), and among those who switched/stopped their original treatment, imputed as non-responders (overall analysis). Logistic regression analysis presenting odds ratios (ORs) and 95% confidence intervals (CIs), including propensity score (PS), stratified on quintiles (inverse probability of treatment weighting as sensitivity analysis) to adjust for imbalanced baseline (BL) covariates.
Results: The overall analysis (n=895) included 439 UST and 456 TNFi-treated pts who had evaluable BL and follow-up data up to 3 years. The remainer analysis included 186 (42.4%) UST and 188 (41.2%) TNFi-treated pts staying on their initial treatment up to 36 ± 3 months. In both the overall and remainer analyses, UST and TNFi groups had significant BL differences in age and psoriasis skin involvement (body surface area >10%); in the overall analysis, significant difference was seen for 1st and 3rd bDMARD line of treatment (Table 1).
In the remainer analysis, both UST and TNFi treatments led to a significant proportion of pts achieving MDA (up to 78%; Figure 1a), VLDA (up to 43%; Figure 1b), cDAPSA LDA (up to 89%; Figure 1c), and cDAPSA remission (up to 64%; Figure 1d). The PS-adjusted ORs (95% CI) indicated a similar response in both cohorts (Figure 1a–d). The overall analysis, which included pts switching/stopping initial treatment during the 3-year period, showed similar results (Figure 2a–d).
Conclusion: In the prospectively followed PsABio study across Europe, slightly more than 40% of pts with PsA stayed on UST or TNFi for 3 years or more. This long-term treatment, when used as 1st-, 2nd-, or 3rd-line bDMARD in a routine care setting, provided sustained improvement of disease signs and symptoms, enabling achievement of MDA, VLDA, cDAPSA LDA, or cDAPSA remission. While numerically more TNFi-treated pts achieved LDA or remission states, UST was used in more treatment-resistant patients and there was no statistical difference between the treatment groups; the improvements were maintained over the 3-year period. The findings demonstrate that remission/LDA are feasible when using targeted drugs in PsA.
 Table 1: Observed baseline characteristics of overall patients (n=895) and remainers (n=374)
Table 1: Observed baseline characteristics of overall patients (n=895) and remainers (n=374)
 Figure 1: Observed proportions of patients and PS-adjusted ORs (95% CI) achieving: (a) MDA; (b) VLDA; (c) cDAPSA LDA; and (d) cDAPSA remission with UST or TNFi up to 3 years (remainer analysis). Results reflect 3-year LOCF data from assessments for patients still under initial treatment at 3 years. *Includes remission. BL, baseline; CI, confidence interval; LOCF, last observation carried forward; LDA, low disease activity; MDA; minimal disease activity; OR, odds ratio; PS, propensity score; TNFi, TNF inhibitor; UST, ustekinumab; VLDA, very low disease activity.
Figure 1: Observed proportions of patients and PS-adjusted ORs (95% CI) achieving: (a) MDA; (b) VLDA; (c) cDAPSA LDA; and (d) cDAPSA remission with UST or TNFi up to 3 years (remainer analysis). Results reflect 3-year LOCF data from assessments for patients still under initial treatment at 3 years. *Includes remission. BL, baseline; CI, confidence interval; LOCF, last observation carried forward; LDA, low disease activity; MDA; minimal disease activity; OR, odds ratio; PS, propensity score; TNFi, TNF inhibitor; UST, ustekinumab; VLDA, very low disease activity.
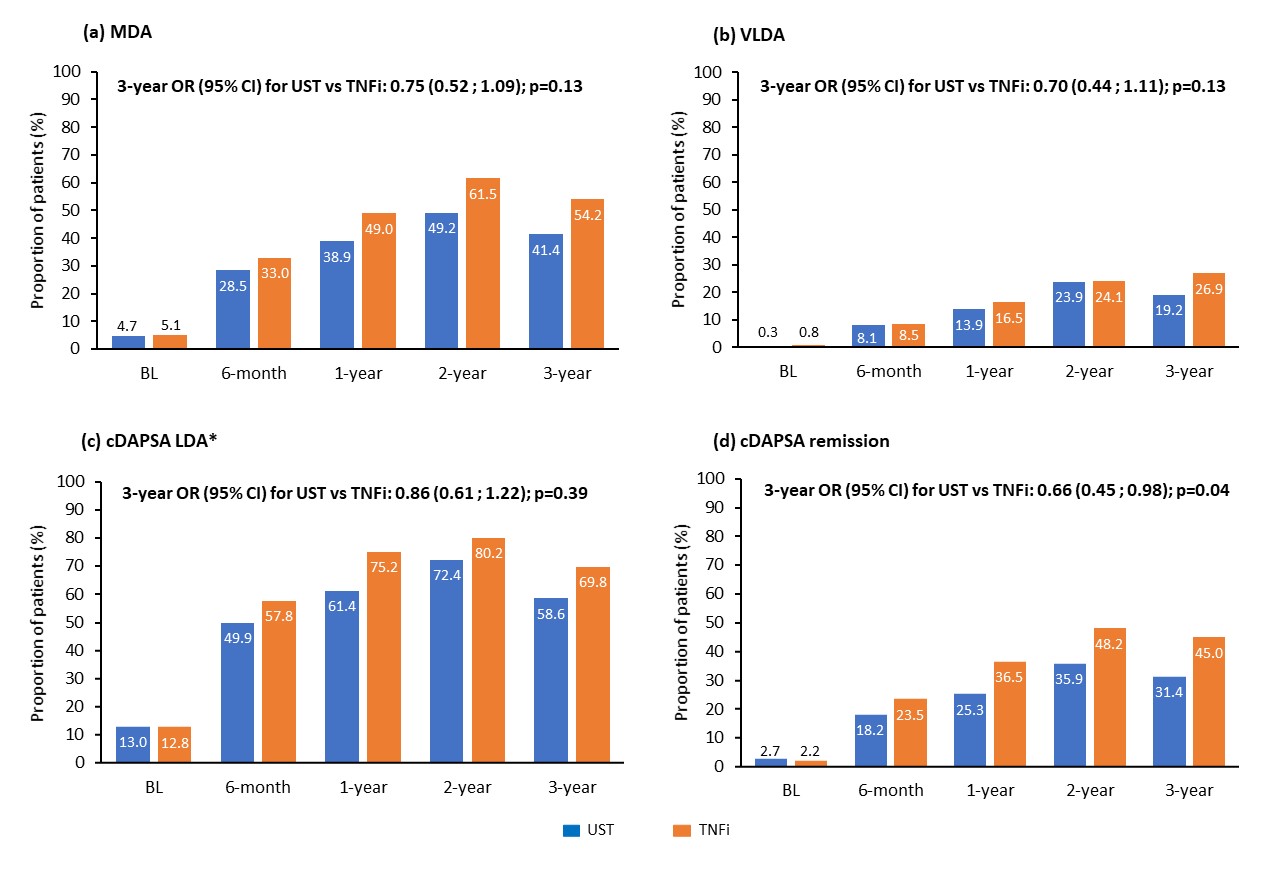 Figure 2: Observed proportions of patients and PS-adjusted ORs (95% CI) achieving: (a) MDA; (b) VLDA; (c) cDAPSA LDA; and (d) cDAPSA remission with UST or TNFi up to 3 years (overall analysis). The overall analysis included patients switching/stopping their original treatment during the 3-year observation period. The PS-adjusted ORs resulting from the overall analysis included non-response imputation in case of stop/switch initial treatment. *Includes remission. BL, baseline; CI, confidence interval; LOCF, last observation carried forward; LDA, low disease activity; MDA; minimal disease activity; OR, odds ratio; PS, propensity score; TNFi, TNF inhibitors; UST, ustekinumab; VLDA, very low disease activity.
Figure 2: Observed proportions of patients and PS-adjusted ORs (95% CI) achieving: (a) MDA; (b) VLDA; (c) cDAPSA LDA; and (d) cDAPSA remission with UST or TNFi up to 3 years (overall analysis). The overall analysis included patients switching/stopping their original treatment during the 3-year observation period. The PS-adjusted ORs resulting from the overall analysis included non-response imputation in case of stop/switch initial treatment. *Includes remission. BL, baseline; CI, confidence interval; LOCF, last observation carried forward; LDA, low disease activity; MDA; minimal disease activity; OR, odds ratio; PS, propensity score; TNFi, TNF inhibitors; UST, ustekinumab; VLDA, very low disease activity.
To cite this abstract in AMA style:
Smolen J, Bergmans P, de Vlam K, Gremese E, Joven-Ibáñez B, Korotaeva T, Noël W, Nurmohamed M, Sfikakis P, Siebert S, Theander E, Gossec L. Sustained Remission/Low Disease Activity Is Feasible in the Long Term in Patients with Psoriatic Arthritis Treated with IL-23/12 Inhibition with Ustekinumab (STELARA®) and Tumor Necrosis Factor Inhibitors in a Real-World, Multicenter Study [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/sustained-remission-low-disease-activity-is-feasible-in-the-long-term-in-patients-with-psoriatic-arthritis-treated-with-il-23-12-inhibition-with-ustekinumab-stelara-and-tumor-necrosis-factor-i/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/sustained-remission-low-disease-activity-is-feasible-in-the-long-term-in-patients-with-psoriatic-arthritis-treated-with-il-23-12-inhibition-with-ustekinumab-stelara-and-tumor-necrosis-factor-i/
