Session Information
Date: Saturday, November 16, 2024
Title: RA – Treatment Poster I
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Quality of life for patients with RA can be severely impacted by pain, fatigue and impaired physical functioning.1 Filgotinib (FIL) has demonstrated early onset improvements in meaningful patient-reported outcomes (PROs) at the group level.2,3 However, it is unknown what proportion of patients have sustained responses at the individual level. This analysis assessed patient-level sustained improvement in pain, fatigue and physical functioning in patients with RA receiving FIL 200 or 100 mg (FIL200/100), adalimumab (ADA) or placebo (PBO).
Methods: This was a post hoc analysis of the Phase 3 trials FINCH 1 (NCT02889796) and FINCH 2 (NCT02873936). In FINCH 1, patients with inadequate response (IR) to methotrexate (MTX) received FIL200/100, ADA or PBO, all with MTX, for 52 weeks (W). In FINCH 2, patients with IR to ≥1 biologic disease-modifying antirheumatic drug (DMARD) received FIL200/100 or PBO, all with conventional synthetic DMARDs, for 24W. Proportions of patients meeting the following response criteria were assessed: “limited to no pain”, “health status not negatively affected by pain”,4 and minimal clinically important differences from baseline in Functional Assessment of Chronic Illness Therapy (FACIT)-Fatigue and Health Assessment Questionnaire–Disability Index (HAQ-DI). Time to and duration of first response were reported by treatment arm. Proportions of responders were estimated using nonresponder imputation, and duration was analyzed using Kaplan–Meier estimates.
Results: Compared with FIL100 or PBO, a greater proportion of patients receiving FIL200 first achieved a response for: “limited to no pain”, “health status not negatively affected by pain”, FACIT-Fatigue and HAQ-DI in FINCH 1 and FINCH 2, except for FACIT-Fatigue with FIL100, for which the proportions were similar. In FINCH 1, a greater proportion of patients in the FIL200 arm reached “limited to no pain” than in the FIL100, ADA or PBO arms by W4 (FIL200: 12.2%, FIL100: 7.1%, ADA: 8.6% vs PBO: 3.6%).
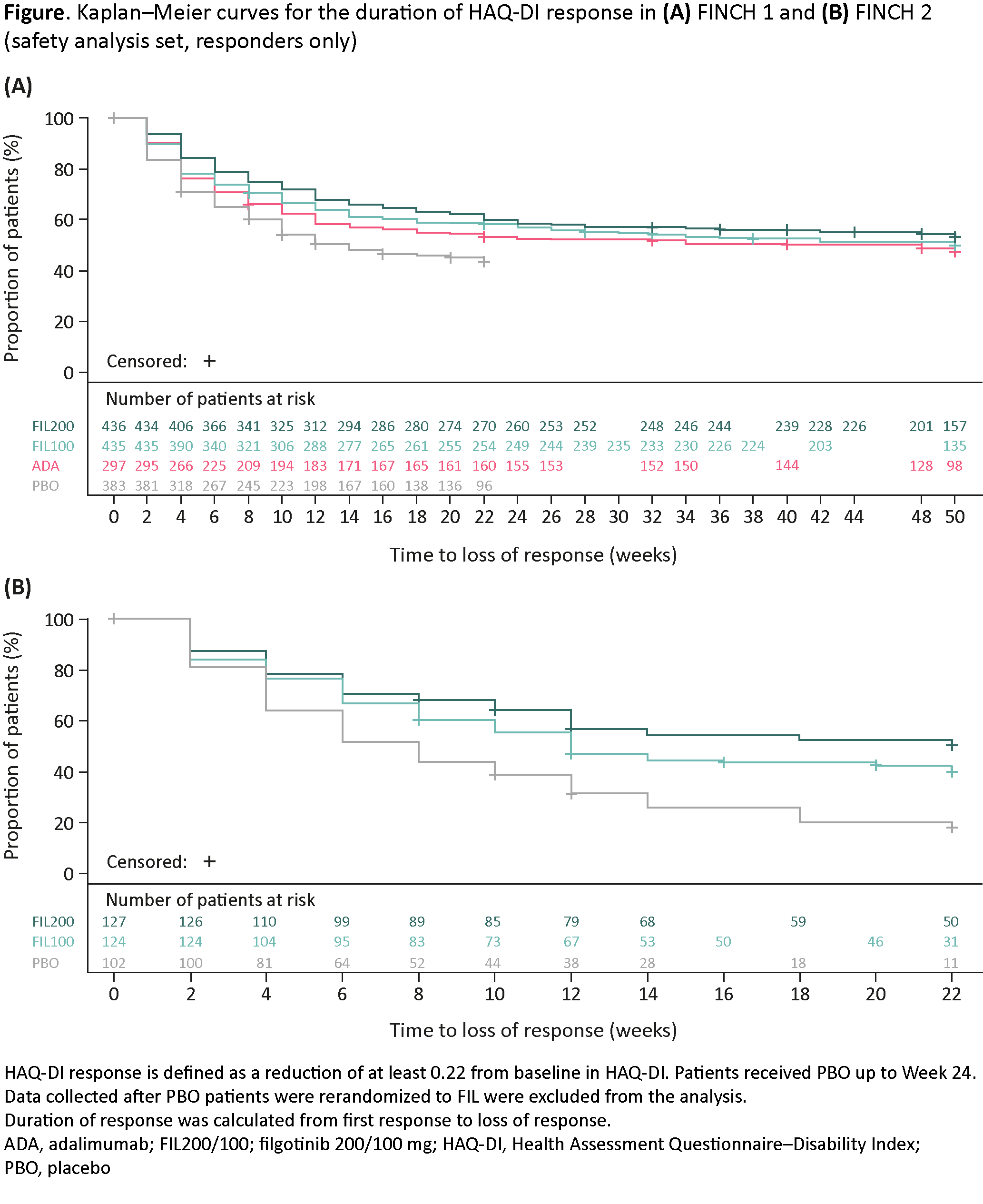
Duration of HAQ-DI response in FINCH 1 and FINCH 2 was similar with FIL200 and FIL100, and was longer than with ADA and PBO (Figure). Loss of HAQ-DI response was observed up to W12, then remained stable. A similar pattern was observed for other PROs in FINCH 1 and FINCH 2, except for FACIT-Fatigue, for which FIL and ADA curves overlapped.
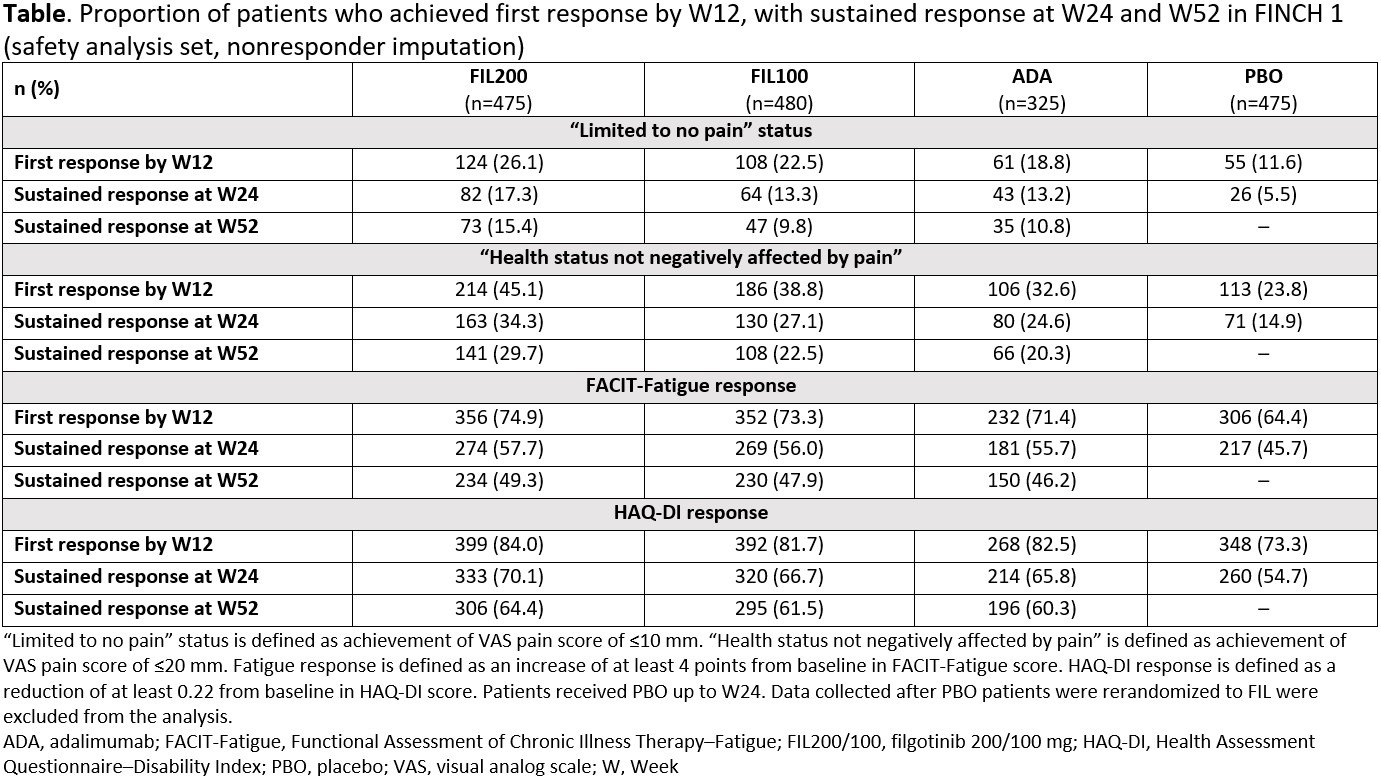
With FIL200, first “limited to no pain” status was achieved by W12 and sustained to W52 in 15.4% of patients in FINCH 1 (Table) and to W24 in 13.6% of patients in FINCH 2. Proportions of patients receiving FIL200 with sustained “health status not negatively affected by pain”, FACIT-Fatigue and HAQ-DI responses were 29.7%, 49.3% and 64.4% in FINCH 1 (W52) and 29.9%, 55.8% and 63.9% in FINCH 2 (W24), respectively.
Conclusion: Meaningful improvements in PROs with FIL in RA at the patient level occur early and are sustained over time. These data strengthen the understanding of the unique clinical and PRO benefits with FIL treatment relevant to patients.
References: 1. Alten R, et al. Arthritis Rheumatol 2023;75(Suppl 9):1680; 2. Taylor PC, et al. RMD Open 2024;10:e003839; 3. Bingham CO 3rd, et al. Arthritis Res Ther 2022;24:11; 4. Taylor PC, et al. J Clin Med 2019;8:831
To cite this abstract in AMA style:
Alten R, Fautrel B, Conaghan P, de Vries D, Faes M, Piovesan M, Van Beneden K, Watson C, Weel-Koenders A, Feist E, de Vlam K. Sustained Patient Meaningful Outcomes of Pain and Fatigue Relief and Improved Physical Functioning with Filgotinib in Rheumatoid Arthritis: A Post Hoc Analysis [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/sustained-patient-meaningful-outcomes-of-pain-and-fatigue-relief-and-improved-physical-functioning-with-filgotinib-in-rheumatoid-arthritis-a-post-hoc-analysis/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/sustained-patient-meaningful-outcomes-of-pain-and-fatigue-relief-and-improved-physical-functioning-with-filgotinib-in-rheumatoid-arthritis-a-post-hoc-analysis/