Session Information
Date: Monday, November 8, 2021
Title: Spondyloarthritis Including PsA – Treatment Poster II: Psoriatic Arthritis I (1329–1363)
Session Type: Poster Session C
Session Time: 8:30AM-10:30AM
Background/Purpose: Bimekizumab (BKZ) is a monoclonal IgG1 antibody that selectively inhibits both interleukin (IL)-17F and IL-17A, and has demonstrated clinical improvements in joint and skin outcomes up to 152 weeks (wks) with an acceptable safety profile in patients (pts) with active psoriatic arthritis (PsA).1,2 Achievement of low disease activity in pts with PsA has been associated with improvements in pt-reported health-related quality of life (HRQoL).3 To report long-term impact of BKZ treatment, up to 3 years, on pt-reported outcome (PRO) measures from a phase 2b dose-ranging study (BE ACTIVE; NCT02969525) and its open-label extension (OLE; NCT03347110).
Methods: BE ACTIVE and OLE study designs have been described previously.1,2 Pts who completed 48 wks of BKZ treatment without meeting withdrawal criteria were eligible for OLE entry. All OLE pts received BKZ 160 mg every 4 wks. Data are presented from BE ACTIVE baseline (BL) and at Wks 48 and 152. PROs reported (full analysis set [FAS]): 9-item Psoriatic Arthritis Impact of Disease (PsAID-9) individual domains (change from BL [CfB]) and Patient Acceptable Symptom State (PASS [score≤4]),4 Health Assessment Questionnaire Disability Index (HAQ-DI) Minimal Clinically Important Difference (MCID; ≥0.35 decrease from BL), CfB in Short Form-36 (SF-36) individual domains, CfB in SF-36 Physical and Mental Component Summaries (PCS/MCS), and CfB in Patient’s Assessment of Arthritis Pain (PtAAP) visual analogue scale (VAS). In addition to observed case (OC) data, we report results with non-responder imputation (NRI) or multiple imputation (MI; based on the missing at random assumption).
Results: Of 206 randomized pts at BL in BE ACTIVE, 66.5% had psoriasis body surface area ≥3%, 18.9% had prior tumor necrosis factor inhibitor exposure, and 63.6% received concomitant methotrexate. Mean BL PRO scores were comparable across dose groups in BE ACTIVE (Table 1). At Wks 48/152, the proportions of patients who achieved PsAID-9 PASS (NRI) were 74.2–76.8%/63.7–67.1%; the proportions of patients who achieved HAQ-DI MCID (NRI) were 47.6–53.7%/42.7–45.1% (Figure 1A–D). All PsAID-9 domains and the majority of the 8 SF-36 domains improved from BL to Wks 48 and 152, including measures of fatigue and bodily pain. SF-36 PCS improved from BL to Wks 48 and 152 and SF-36 MCS remained within the normal range from BL, as expected (Figure 1E–F; Table 2). Pts showed sustained improvements in pain by mean CfB in PtAAP VAS score at Wk 48 (between −26.8 and −34.7; MI) and Wk 152 (between −31.3 and −33.0; MI) (Figure 1G). There were no clear differences between pts who completed either BKZ 160 mg or BKZ 320 mg in BE ACTIVE prior to receiving BKZ 160 mg in the OLE.
Conclusion: BKZ treatment is associated with sustained improvements in PRO measures of disease impact up to 3 years, including in pain and fatigue. Furthermore, BKZ treatment is associated with substantial and durable improvements in measures of overall physical function and HRQoL up to 3 years.
References: 1. Ritchlin CT. Lancet 2020;395:427–40; 2. McInnes IB. Ann Rheum Dis 2020;79:1153–4; 3. Gossec L. Ann Rheum Dis 2020;79:1687; 4. Gossec L. Ann Rheum Dis 2014;73:1012–9.
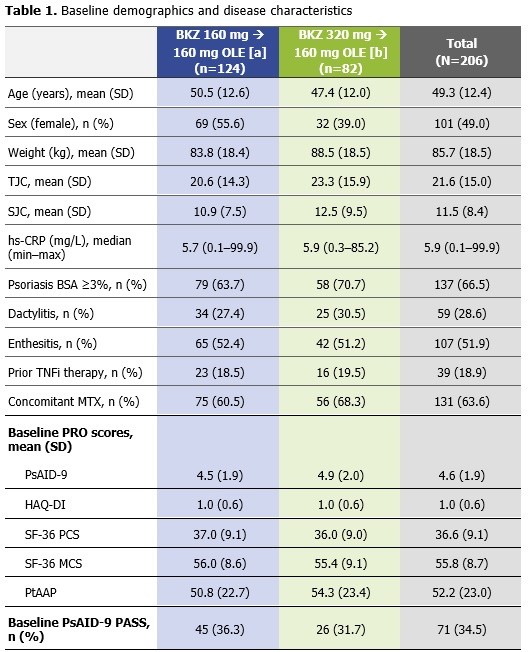 Full analysis set. All patients received BKZ 160 mg during the OLE (Wks 48–152) after completing BKZ 160 mg or 320 mg in BE ACTIVE: [a] Includes pts within the indicated analysis set from the dose-response, double-blind period who were subsequently re-randomized to 160 mg, or [b] pts who received 320 mg during the dose-blind periods; all pts received 160 mg during the open-label extension. Lower HAQ-DI scores correspond to better performance of daily activities; Higher SF_36 scores indicate better pt HRQoL; Lower PsAID-9 scores correspond to lower impact of disease on pt QoL; Lower PtAAP VAS scores indicate less pain. BKZ: bimekizumab; BSA: body surface area; HAQ-DI: Health Assessment Questionnaire Disability Index; hs-CRP: high sensitivity C-reactive protein; MTX: methotrexate; OLE: open-label extension; PASS: Patient Acceptable Symptom State; PRO: patient-reported outcome; PsAID-9: Psoriatic Arthritis Impact of Disease-9; PtAAP: Patient’s Assessment of Arthritis Pain; pts: patients; (HR)QoL: (health-related) quality of life; SD: standard deviation; SF_36 MCS: Short Form_36 Mental Component Summary; SF_36 PCS: Short Form_36 Physical Component Summary; SJC: swollen joint count; TJC: tender joint count; TNF: tumor necrosis factor; VAS: visual analogue scale; wk: week.
Full analysis set. All patients received BKZ 160 mg during the OLE (Wks 48–152) after completing BKZ 160 mg or 320 mg in BE ACTIVE: [a] Includes pts within the indicated analysis set from the dose-response, double-blind period who were subsequently re-randomized to 160 mg, or [b] pts who received 320 mg during the dose-blind periods; all pts received 160 mg during the open-label extension. Lower HAQ-DI scores correspond to better performance of daily activities; Higher SF_36 scores indicate better pt HRQoL; Lower PsAID-9 scores correspond to lower impact of disease on pt QoL; Lower PtAAP VAS scores indicate less pain. BKZ: bimekizumab; BSA: body surface area; HAQ-DI: Health Assessment Questionnaire Disability Index; hs-CRP: high sensitivity C-reactive protein; MTX: methotrexate; OLE: open-label extension; PASS: Patient Acceptable Symptom State; PRO: patient-reported outcome; PsAID-9: Psoriatic Arthritis Impact of Disease-9; PtAAP: Patient’s Assessment of Arthritis Pain; pts: patients; (HR)QoL: (health-related) quality of life; SD: standard deviation; SF_36 MCS: Short Form_36 Mental Component Summary; SF_36 PCS: Short Form_36 Physical Component Summary; SJC: swollen joint count; TJC: tender joint count; TNF: tumor necrosis factor; VAS: visual analogue scale; wk: week.
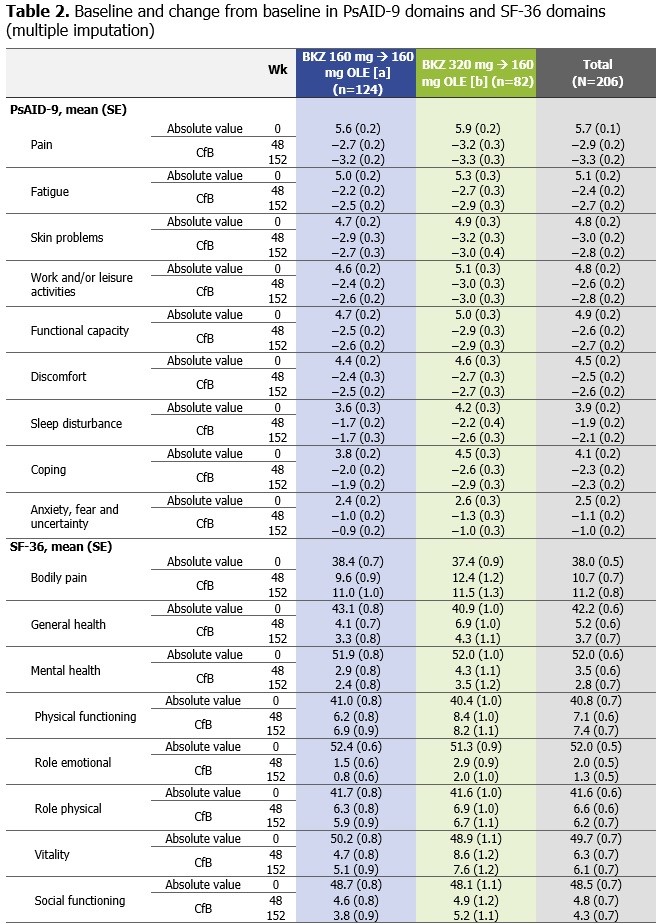 Full analysis set. All patients received BKZ 160 mg during the OLE (Wks 48–152) after completing BKZ 160 mg or 320 mg in BE ACTIVE: [a] Includes pts within the indicated analysis set from the dose-response, double-blind period who were subsequently re-randomized to 160 mg, or [b] pts who received 320 mg during the dose-blind periods; all pts received 160 mg during the open-label extension. Higher SF_36 scores indicate better pt HRQoL; Lower PsAID-9 scores correspond to lower impact of disease on pt QoL. Missing data were imputed using multiple imputation based on the Markov-Chain Monte Carlo method for the intermittent missing data, followed by monotone regression for the monotone missing data assuming missing at random. BKZ: bimekizumab; BL: baseline; CfB: change from Baseline; OLE: open-label extension; PsAID-9: Psoriatic Arthritis Impact of Disease-9; pts: patients; (HR)QoL: (health-related) quality of life; SE: standard error; SF_36: Short Form_36; wk: week.
Full analysis set. All patients received BKZ 160 mg during the OLE (Wks 48–152) after completing BKZ 160 mg or 320 mg in BE ACTIVE: [a] Includes pts within the indicated analysis set from the dose-response, double-blind period who were subsequently re-randomized to 160 mg, or [b] pts who received 320 mg during the dose-blind periods; all pts received 160 mg during the open-label extension. Higher SF_36 scores indicate better pt HRQoL; Lower PsAID-9 scores correspond to lower impact of disease on pt QoL. Missing data were imputed using multiple imputation based on the Markov-Chain Monte Carlo method for the intermittent missing data, followed by monotone regression for the monotone missing data assuming missing at random. BKZ: bimekizumab; BL: baseline; CfB: change from Baseline; OLE: open-label extension; PsAID-9: Psoriatic Arthritis Impact of Disease-9; pts: patients; (HR)QoL: (health-related) quality of life; SE: standard error; SF_36: Short Form_36; wk: week.
 Full analysis set. All patients received BKZ 160 mg during the OLE (Wks 48–152) after completing BKZ 160 mg or 320 mg in BE ACTIVE: [a] Includes pts within the indicated analysis set from the dose-response, double-blind period who were subsequently re-randomized to 160 mg, or [b] pts who received 320 mg during the dose-blind periods; all pts received 160 mg during the open-label extension; [c] OC baseline [160 mg/320 mg/Total]: n=124/82/206, respectively; Wk 48 [160 mg/320 mg/Total]: n=115/76/191, respectively; Wk 152 [160 mg/320 mg/Total]: n=95/62/157, respectively; [d] OC Wk 48 [160 mg/320 mg/Total]: n=115/76/191, respectively; Wk 152 [160 mg/320 mg/Total]: n=95/62/157, respectively. Higher SF_36 scores indicate better pt HRQoL; Lower PtAAP VAS scores indicate less pain. BKZ: bimekizumab; HAQ-DI: Health Assessment Questionnaire Disability Index; MCID: minimal clinically important difference; MI: multiple imputation; NRI: non-responder imputation; OC: observed case; OLE: open-label extension; PASS: Patient Acceptable Symptom State; PsAID-9: Psoriatic Arthritis Impact of Disease-9; PtAAP: Patient’s Assessment of Arthritis Pain; pts: patients; (HR)QoL: (health-related) quality of life; SF_36 MCS: Short Form_36 Mental Component Summary; SF_36 PCS: Short Form_36 Physical Component Summary; VAS: visual analogue scale; wk: week.
Full analysis set. All patients received BKZ 160 mg during the OLE (Wks 48–152) after completing BKZ 160 mg or 320 mg in BE ACTIVE: [a] Includes pts within the indicated analysis set from the dose-response, double-blind period who were subsequently re-randomized to 160 mg, or [b] pts who received 320 mg during the dose-blind periods; all pts received 160 mg during the open-label extension; [c] OC baseline [160 mg/320 mg/Total]: n=124/82/206, respectively; Wk 48 [160 mg/320 mg/Total]: n=115/76/191, respectively; Wk 152 [160 mg/320 mg/Total]: n=95/62/157, respectively; [d] OC Wk 48 [160 mg/320 mg/Total]: n=115/76/191, respectively; Wk 152 [160 mg/320 mg/Total]: n=95/62/157, respectively. Higher SF_36 scores indicate better pt HRQoL; Lower PtAAP VAS scores indicate less pain. BKZ: bimekizumab; HAQ-DI: Health Assessment Questionnaire Disability Index; MCID: minimal clinically important difference; MI: multiple imputation; NRI: non-responder imputation; OC: observed case; OLE: open-label extension; PASS: Patient Acceptable Symptom State; PsAID-9: Psoriatic Arthritis Impact of Disease-9; PtAAP: Patient’s Assessment of Arthritis Pain; pts: patients; (HR)QoL: (health-related) quality of life; SF_36 MCS: Short Form_36 Mental Component Summary; SF_36 PCS: Short Form_36 Physical Component Summary; VAS: visual analogue scale; wk: week.
To cite this abstract in AMA style:
Gossec L, Asahina A, Gottlieb A, Coates L, Ink B, Assudani D, Coarse J, Hellot S, Eells J, Mease P. Sustained Improvement in Physical Function, Disease Impact and Health-Related Quality of Life in Patients with Psoriatic Arthritis Treated with Bimekizumab: 3-Year Results from a Phase 2b Open-Label Extension Study [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/sustained-improvement-in-physical-function-disease-impact-and-health-related-quality-of-life-in-patients-with-psoriatic-arthritis-treated-with-bimekizumab-3-year-results-from-a-phase-2b-open-l/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/sustained-improvement-in-physical-function-disease-impact-and-health-related-quality-of-life-in-patients-with-psoriatic-arthritis-treated-with-bimekizumab-3-year-results-from-a-phase-2b-open-l/
