Session Information
Session Type: Poster Session B
Session Time: 9:00AM-10:30AM
Background/Purpose: The sustainability of the immunogenicity of BNT162b2 anti-severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccine in patients with autoimmune rheumatic diseases (ARDs) receiving immunomodulators is uncertain. The Objective of this study is to evaluate whether the durability of the humoral immune response to BNT162b2 vaccine differs between patients without and with ARDs receiving immunomodulators.
Methods: We retrospectively reviewed the electronic medical records of patients with ARDs and control patients without ARDs. Patients who were SARS-CoV-2 infection-naïve, had completed COVID19 vaccination using, had been serologically tested for anti-SARS-CoV-2 S immunoassays using Elecsys® at least 6 months post second dose of the vaccine and had not received the third booster dose were included in the analysis. The overall mean of anti-SARS-CoV-2 S protein titer and at two cut offs; < 250 and < 132 binding antibody unit/mL (BAU/mL) were compared between the two groups using unpaired t-test and Chi-square test. Binary logistic regression analysis was performed to identify which immunomodulators associated with a significant drop in the antibody level.
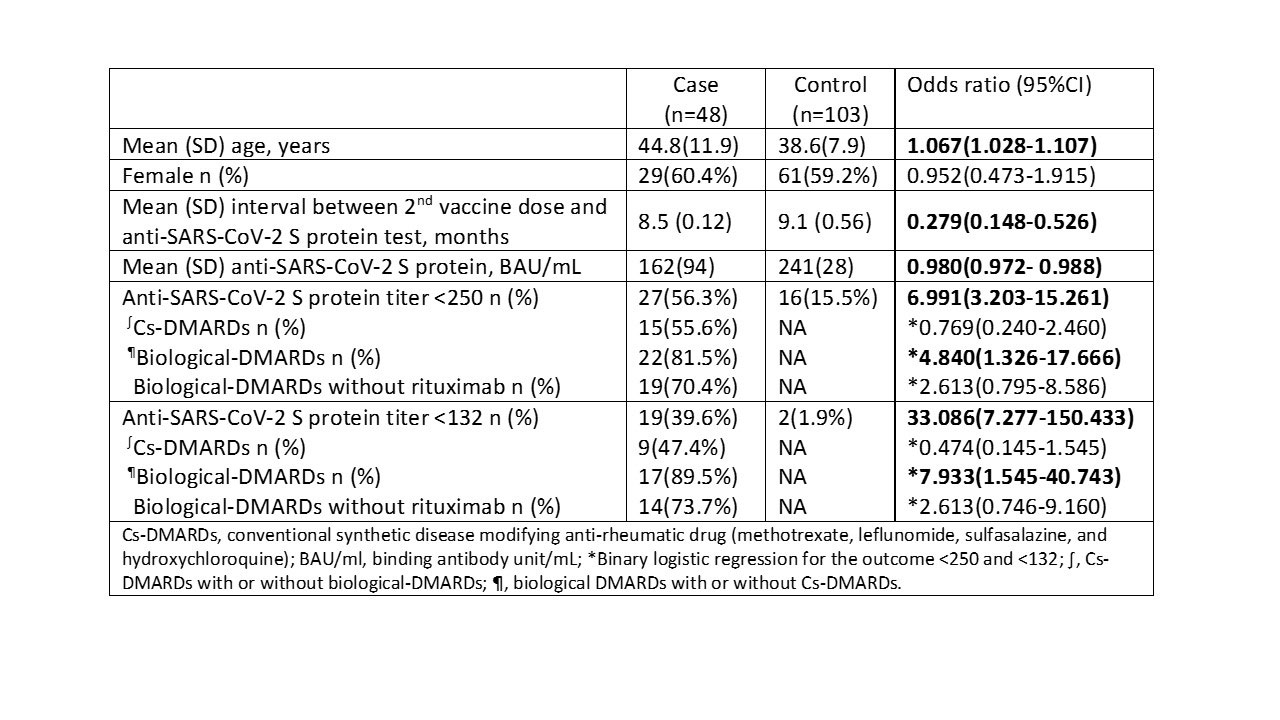
Results: Mean (SD) ages of 48 patients with ARDs and 103 controls were 44.8 (11.9) vs. 38.6 (7.9) years. Women predominated in both groups (60% vs. 59%, P = 0.889). The most frequently prescribed disease modifying anti-rheumatic drug (DMARDs) was TNF-i in 24 (50%) followed by methotrexate in 22 (45.8%), hydroxychloroquine in 10 (20.8%), lL6-i in 4 (8.3%), and rituximab in 3 (6.3%) patients. Six months post vaccination, the overall mean (SD) anti-SARS-CoV-2 S antibody titer in the case group was significantly lower than the controls; 162 (94) vs. 241 (28), OR (95%CI) 0.980(0.972- 0.988). At both cut offs (< 250 and < 132 BAU/mL), the number of cases whose antibody dropped below the cut offs was significantly lower in ARDs group compared to controls; 27 (56.3%) vs. 16 (15.5%), P < 0.001 and 19 (39.6%) vs. 2 (1.9%), P < 0.001, respectively. Nevertheless, neither conventional synthetic-DMARDs nor biological-DMARDs (without rituximab) was associated with a significant drop in antibody titer at the two cut offs (Table 1).
Conclusion: Immunogenicity induced by BNT162b2 vaccine in patients with ARDs receiving immunomodulators fades faster than in patients without ARDs. Cs-DMARDs and biological-DMARDs (without rituximab) may not play a role in decreasing the durability of immunogenicity, however a study with larger sample size is needed to confirm this.
To cite this abstract in AMA style:
Alsaed O, Chaponda M, Satti E, Ashour H, Almaslamani M, Al emadi S. Sustainability of Immunogenicity of BNT162b2 Vaccine in Patients with Autoimmune Rheumatic Disease, a Retrospective Comparative Study [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/sustainability-of-immunogenicity-of-bnt162b2-vaccine-in-patients-with-autoimmune-rheumatic-disease-a-retrospective-comparative-study/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/sustainability-of-immunogenicity-of-bnt162b2-vaccine-in-patients-with-autoimmune-rheumatic-disease-a-retrospective-comparative-study/