Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose: Cartilage damage is a key feature of inflammatory and degenerative arthritis and an important determinant of patient outcomes. Chondrocytes are the only cells in articular cartilage and are critical for tissue homeostasis. Relatively little is known about how chondrocyte responses to cytokines are regulated. Suppressor of cytokine signaling-3 (SOCS-3) is a negative regulator of the IL-6 cytokine family, which utilizes the common gp130 signaling receptor, in other cell types. We investigated the impact of gp130 cytokines on cartilage and isolated chondrocytes and examined the impact of SOCS-3 deficiency on chondrocytes, in vitro and in vivo.
Methods: Conditional gene targeting was used to generate mice lacking SOCS-3 in chondrocytes by crossing mice with a floxed allele of SOCS-3, with mice transgenic for Cre recombinase driven by the type II collagen (Col2a1) promoter (Socs3Δ/Δcol2 mice). Primary chondrocytes and femoral head cartilage explants were obtained from control and Socs3Δ/Δcol2 mice. Cartilage explant cultures were stimulated with gp130 cytokines and aggrecanase activity measured using the 1,9-DMMB dye-binding assay and quantitative PCR. STAT1, STAT3 and STAT5 phosphorylation and cytokine/chemokine production in response to gp130 cytokines were measured by flow cytometry and multiplex bead array, respectively. The responses of littermate and Socs3Δ/Δcol2 mice to intra-articular injections of gp130 cytokines and to K/BxN serum transfer arthritis were assessed clinically and histologically.
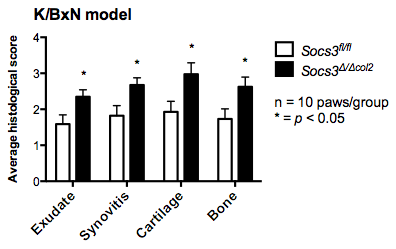
Results: In wild-type articular chondrocytes, all gp130 cytokines activated JAK-STAT signaling and upregulated Socs3 mRNA expression. Stimulation with Oncostatin M caused the most STAT phosphorylation. Socs3Δ/Δcol2 cartilage explants and isolated chondrocytes stimulated with gp130 cytokines exhibited prolonged STAT1, STAT3 and STAT5 activation, enhanced Adamts4 and Adamts5 production and cartilage degradation. In addition, SOCS-3 deletion in chondrocytes increased the production of multiple inflammatory mediators (IL-6, G-CSF, CXCL1 and CCL2), including receptor activator of NF-kB ligand (RANKL). All features of joint pathology in the acute inflammatory arthritis model – exudate, synovitis, cartilage degradation and bone erosion – were exacerbated in Socs3Δ/Δcol2 mice compared to Socs3fl/fl littermate controls (see figure). Total histological scores were 9.5±0.78 vs. 6.0±0.96 (p=0.006), for Socs3Δ/Δcol2 and Socs3fl/fl mice, respectively. Furthermore, microarray analysis showed profound and selective effects on gene expression in response to gp130 cytokines, which were heightened in the absence of SOCS3.
Conclusion: Our findings highlight important biological effects of gp130 cytokines on cartilage and provide the first direct evidence for a key regulatory role of SOCS-3 in articular chondrocytes.
Disclosure:
X. Liu,
None;
K. E. Lawlor,
None;
B. A. Croker,
None;
I. P. Wicks,
None.
« Back to 2013 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/suppressor-of-cytokine-signaling-3-is-a-critical-regulator-of-gp130-cytokine-signaling-in-articular-chondrocytes/