Session Information
Session Type: Poster Session A
Session Time: 1:00PM-3:00PM
Background/Purpose: Immunosuppressive states can attenuate the immunogenicity of the COVID-19 vaccines. Some studies have shown an improved immune response with a supplemental primary dose. In August 2021, the CDC recommended that immunocompromised individuals (including patients on immunosuppressive agents) receive a supplemental primary dose of the vaccine ≥28 days after their second dose of the Pfizer BioNTech or Moderna COVID-19 vaccine. The American College of Rheumatology Covid-19 vaccine task force strongly supports these recommendations, as well as a supplemental dose of the Johnson and Johnson vaccine. This study aims to determine how many patients on immunosuppressive Disease Modifying Anti-Rheumatic Drugs (DMARDs) and biologic agents have received a supplemental dose of the COVID-19 vaccine in a resource-limited population in rural southeast United States of America. We aim to determine underlying barriers to obtaining adequate vaccination.
Methods: This retrospective chart review and questionnaire included patients seen in our rheumatology and internal medicine clinic from November 1, 2021 to February 28, 2022. Patients included in the study were on any of the following medications; Methotrexate, Mycophenolate, Azathioprine, Leflunomide, Etanercept, Adalimumab, Certolizumab, Golimumab, Tocilizumab, Abatacept, Rituximab, and Infliximab, from at least July 1, 2021 to the time of study. Demographics, diagnosis, and COVID-19 vaccination status were collected from patient records. Then a short telephone questionnaire was administered. Descriptive analysis including Chi-square test and Fishers exact test were used.
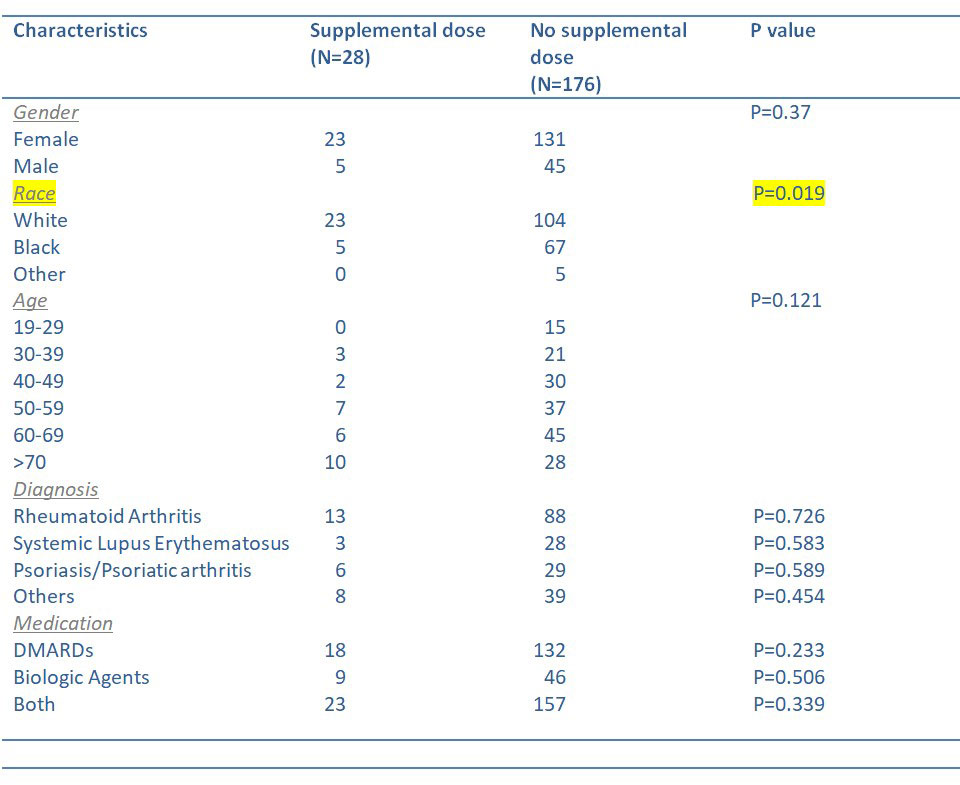
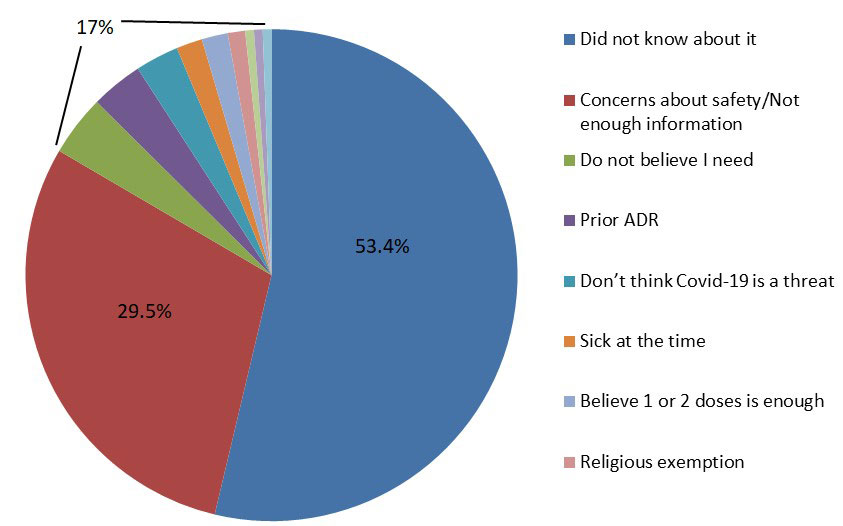
Results: A total of 204 patients were included. Twenty eight patients (13.7%) received a supplemental dose and 176 (86.3%) did not. Of those 176 patients, 94 (53.4%) did not know about this recommendation, 52 (29.5%) had safety concerns or felt that they did not have enough information, and 30 (17%) patients had other reasons including prior adverse effects, religious exemption, or belief that it is not needed. Out of the total subjects, 55 (27 %) were unvaccinated while 121 (59.3%) received partial vaccination and 107 (52.4%) received the booster vaccine. There was no association of supplemental vaccination rate to age, gender, vaccine type, diagnosis, or medication. White patients were more likely to have received the supplemental primary dose (p= 0.019) compared to other races.
Conclusion: This study reveals that most immunosuppressed patients with autoimmune conditions in our resource-limited rural population have not received the supplemental primary dose of the COVID-19 vaccine. The most common reason was lack of knowledge of vaccine recommendations. This could be due to frequent guideline changes, patient education, and even healthcare provider education. Most patients with safety concerns did not receive any doses. Other reasons included adverse reactions, religious exemption, and belief that COVID-19 is not a threat. There could be similar patterns in other comparable populations. It is important to educate providers and patients about appropriate vaccination regimen, as the immunosuppressed are more vulnerable to COVID-19 infection and its most severe complications.
To cite this abstract in AMA style:
Murugan A, Ruiz X, Harris Jr. L. Supplemental Primary Dose of the COVID-19 Vaccine in Immunosuppressed Patients on DMARDs and Biologic Agents [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/supplemental-primary-dose-of-the-covid-19-vaccine-in-immunosuppressed-patients-on-dmards-and-biologic-agents/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/supplemental-primary-dose-of-the-covid-19-vaccine-in-immunosuppressed-patients-on-dmards-and-biologic-agents/