Session Information
Date: Tuesday, November 14, 2023
Title: (2039–2060) Pediatric Rheumatology – Clinical Poster III: Potpourri
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: Childhood Chronic Idiopathic Uveitis (cCIU) is a severe ocular condition that accounts for the 40% of all uveitis in children. Its timely and proper treatment is critical to prevent severe complications. Our purpose isto report the treatment response of a large cohort of cCIU.
Methods: This is a retrospective multicentre international observation study conducted at Meyer Children’s Hospital IRCCS and Bristol Royal Hospital for children. The medical records of children were reviewed if they have a diagnosis of cCIU prior to 16 years old, and received a systemic treatment other than corticosteroids. We collected demographic, clinical and laboratory data. The main outcome was the achievement of response and remission on treatment according to SUN criteria.
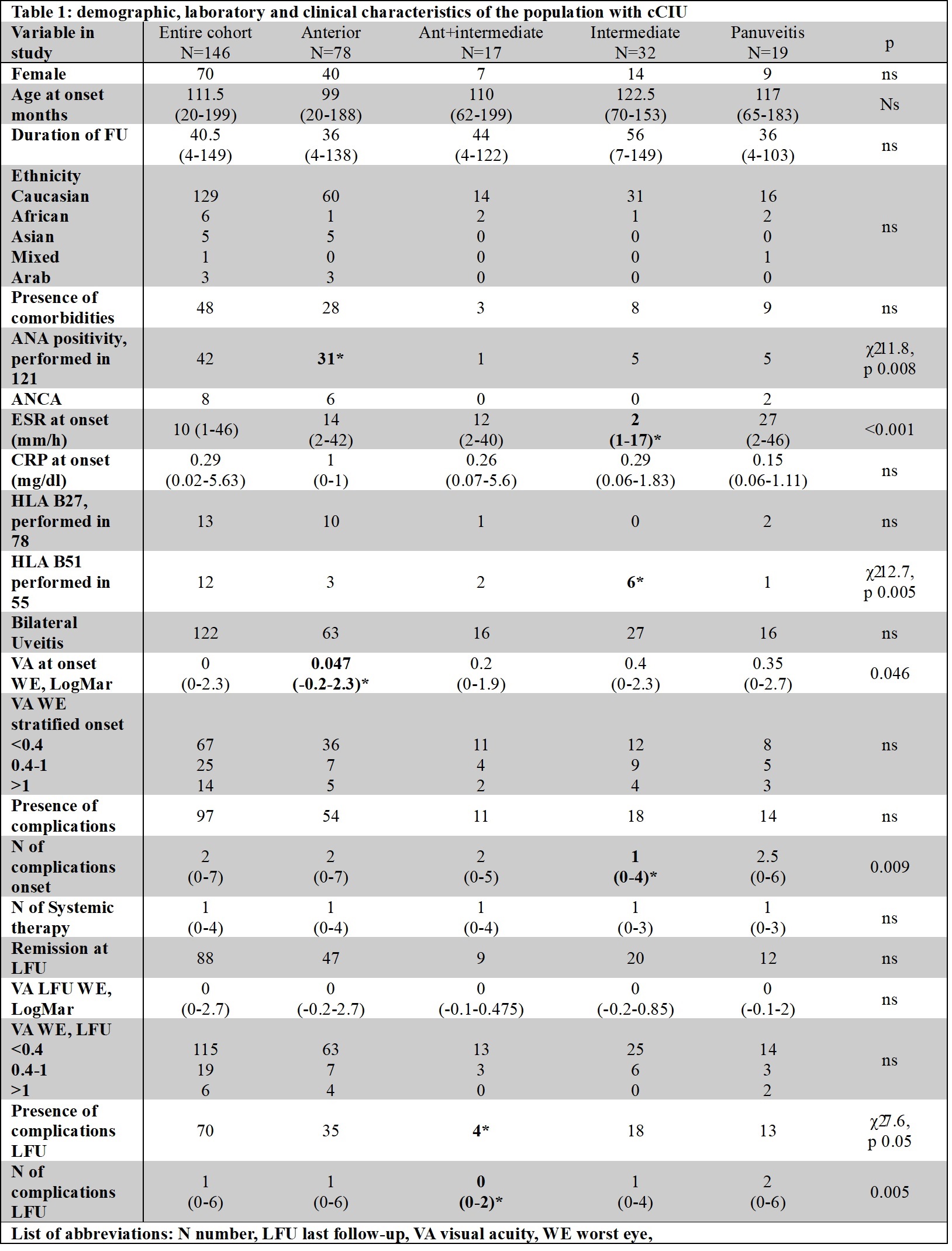
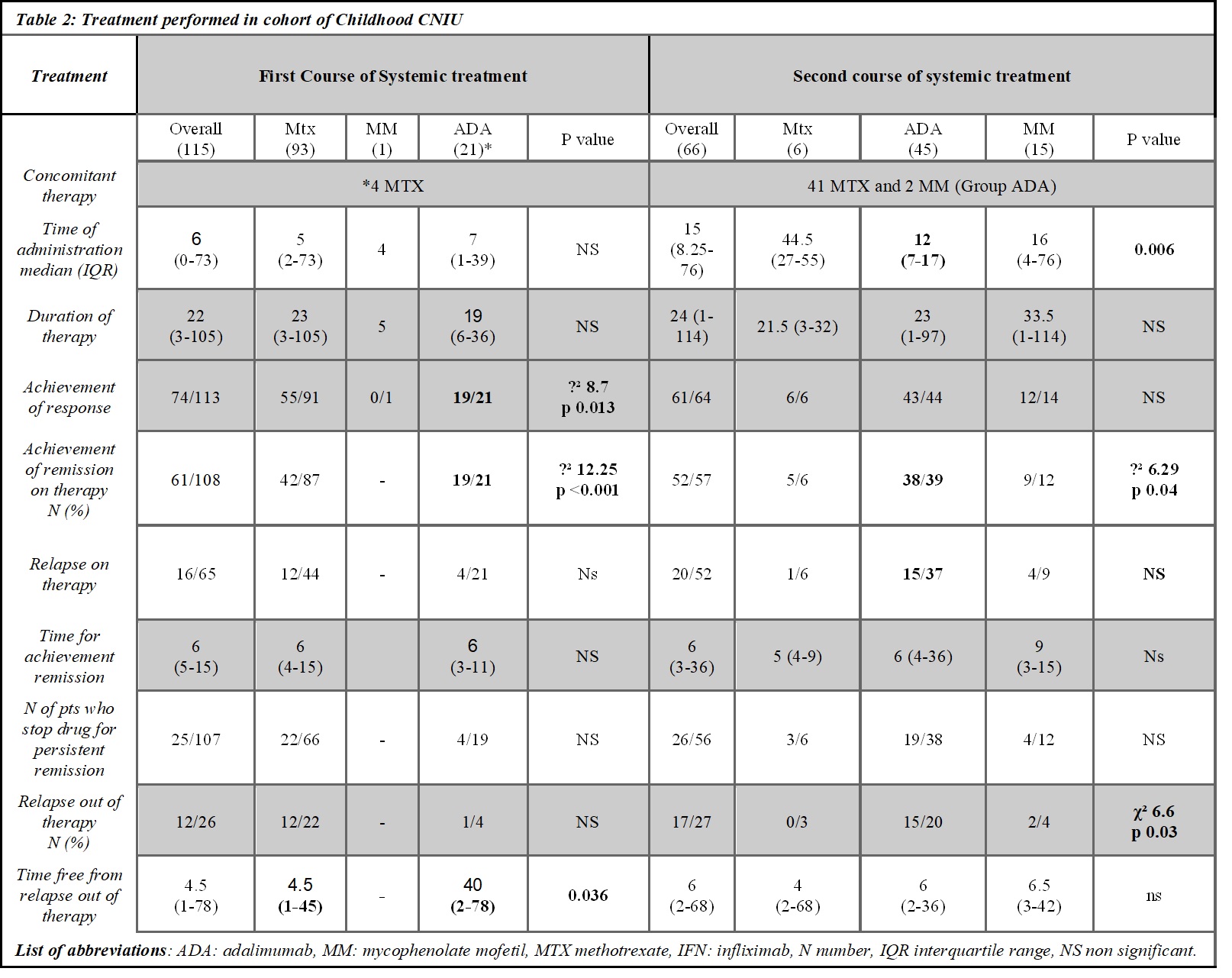
Results: 146 cCIU (76 male, 42 ANA positive) with a median age at onset of 112months (range 20-199), 122 had a bilateral involvement (83.6%), 78 had anterior uveitis (53.4%), 17 anterior+intermediate (11.6%), 32 intermediate (21.9%) and 19 panuveitis (13%) were screened for inclusion criteria (see table 1). 115 patients received at least one systemic therapy other than corticosteroids (78.8%), 68 two different lines (46.6%), 20 three-line treatment (13.7%), and 3 four-line treatment (2.1). The median time for the first drug administered was 6 months (range 0-73). As first line-treatment 93 children received methotrexate (63.7%), 21 adalimumab (14.4%) (of whom 4 with concomitant methotrexate), and 1 mycophenolate mofetil (MMF) (0.7%). As second line treatment 6 received methotrexate, 45 adalimumab, 15 MMF, 1 azathioprine, and 1 tacrolimus. Among the first-line therapies, we observed that patients treated with adalimumab achieved more frequently ocular response (19/21 vs 55/91 χ² 8.72 p 0.01) and ocular remission (19/21 vs 42/45 χ² 12.2 p < 0.001), with no difference in frequency of relapse on therapy and time to first relapse on the therapy (see table 2). Additionally, cCIU with worse visual acuity ( >0.4 LogMAR) were less likely to achieve disease remission with methotrexate as first-line treatment (χ² 6.7p 0.035). Among the second-line therapy, adalimumab achieved more frequently ocular remission (38/1 (ADA) vs 5/1 (MTX) vs 9/12 (MMF), χ² 6.29 p 0.043) (table 2).
Conclusion: Adalimumab showed better chance to achieve ocular remission, as first and second line treatment, as well as in patients with worst outcome: ANA positivity, worse visual acuity, intermediate uveitis. Acknowledgements: All the patients that received adalimumab as first line treatment were followed at Meyer Children’s Hospital IRCCS, and the off label prescription authorization was obtained.
To cite this abstract in AMA style:
Maccora I, Guly C, Sanfilippo L, Soldovieri s, De Libero C, Ramanan A, Simonini G. Superiority of Adalimumab in Treating Childhood Chronic Idiopathic Uveitis: Evidence from a Multicentre Experience [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/superiority-of-adalimumab-in-treating-childhood-chronic-idiopathic-uveitis-evidence-from-a-multicentre-experience/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/superiority-of-adalimumab-in-treating-childhood-chronic-idiopathic-uveitis-evidence-from-a-multicentre-experience/