Session Information
Session Type: Abstract Session
Session Time: 4:00PM-5:30PM
Background/Purpose: Rituximab (RTX) treatment significantly decreases the antibody response to COVID-19 vaccines, raising concerns about its use. However, unlike during the pandemic, most patients initiating RTX now, will receive COVID-19 vaccination before initiating RTX. Thus, the effect of RTX treatment on serological vaccine response in pre-vaccinated individuals remains unclear. We aimed to assess the antibody (abs) level and function by three different SARS-CoV-2 abs assays in COVID-19 vaccinated patients, depending on the timing of RTX treatment initiation, either before or after vaccination.
Methods: Patients treated with RTX at the Dep. of Rheumatology, Aarhus University Hospital, from 2017 to 2022 were eligible for inclusion (n=368). Patients were categorized based on the order of RTX treatment to their primary COVID-19 vaccination: RTX pre (RTX only prior to vaccination), RTX pre+post (RTX prior to and after vaccination), and RTX post (RTX only after vaccination). Additionally, we investigated the effect of vaccines administered ≥ 9 months since the last RTX treatment, “RTX-free” vaccinations. Serological response was evaluated by measuring 2 different SARS-CoV-2-IgGs (Spike and Nucleocapsid) and the concentration and activity of neutralizing antibodies. Blood donors (BDs) were enrolled as controls, n=113.
Results: We included 254 patients; 67% female, aged 62 years. The predominant diagnoses were ANCA-vasculitis (31%), rheumatoid arthritis (26%) and myositis (12%). Patients had on average four COVID-19 vaccinations and one infection, while BDs had respectively three and one.
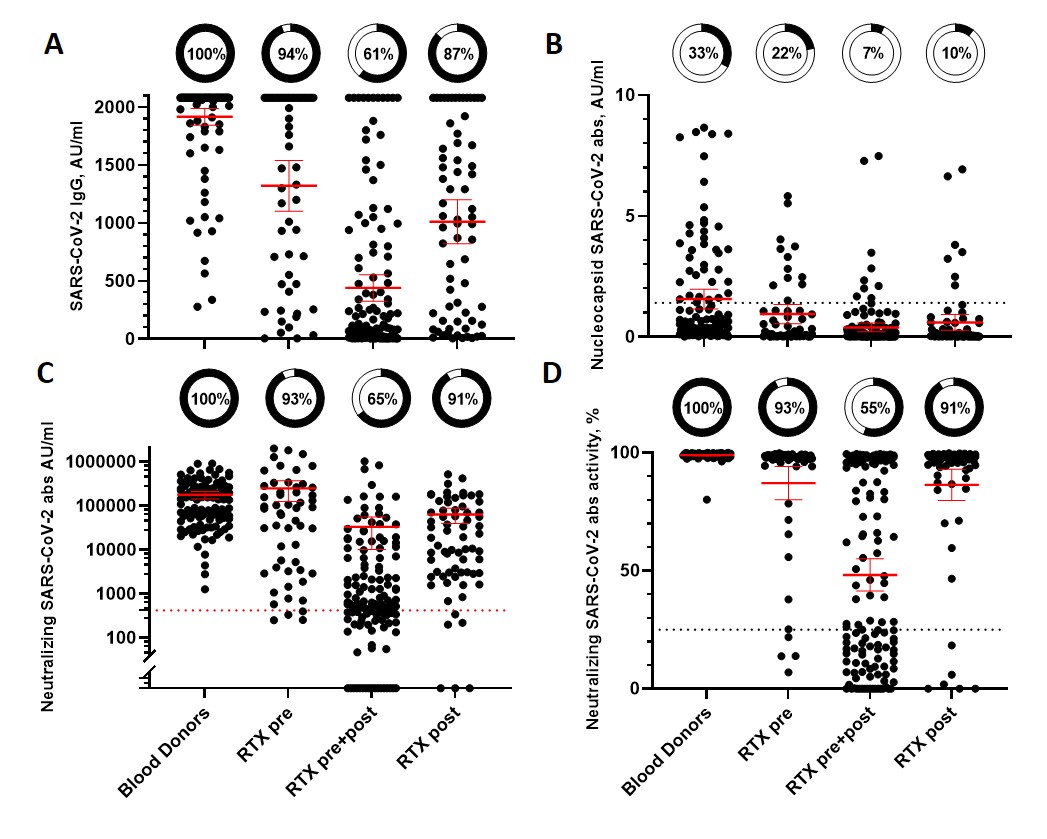
The RTX pre+post group (n=132) had significantly lower concentrations of spike and neutralizing abs, neutralizing activity and seroconversion compared to the RTX pre (n=54) and RTX postgroup(n=68), Fig 1A-D. Only 65% of RTX pre+postdeveloped neutralizing abs compared to 91% for theRTX post and 93% for theRTX pre, Fig 1C. There was no difference between the RTX pre or RTX post in seroconversion of the 3 abs measured (all p’s ≥ 0.08).
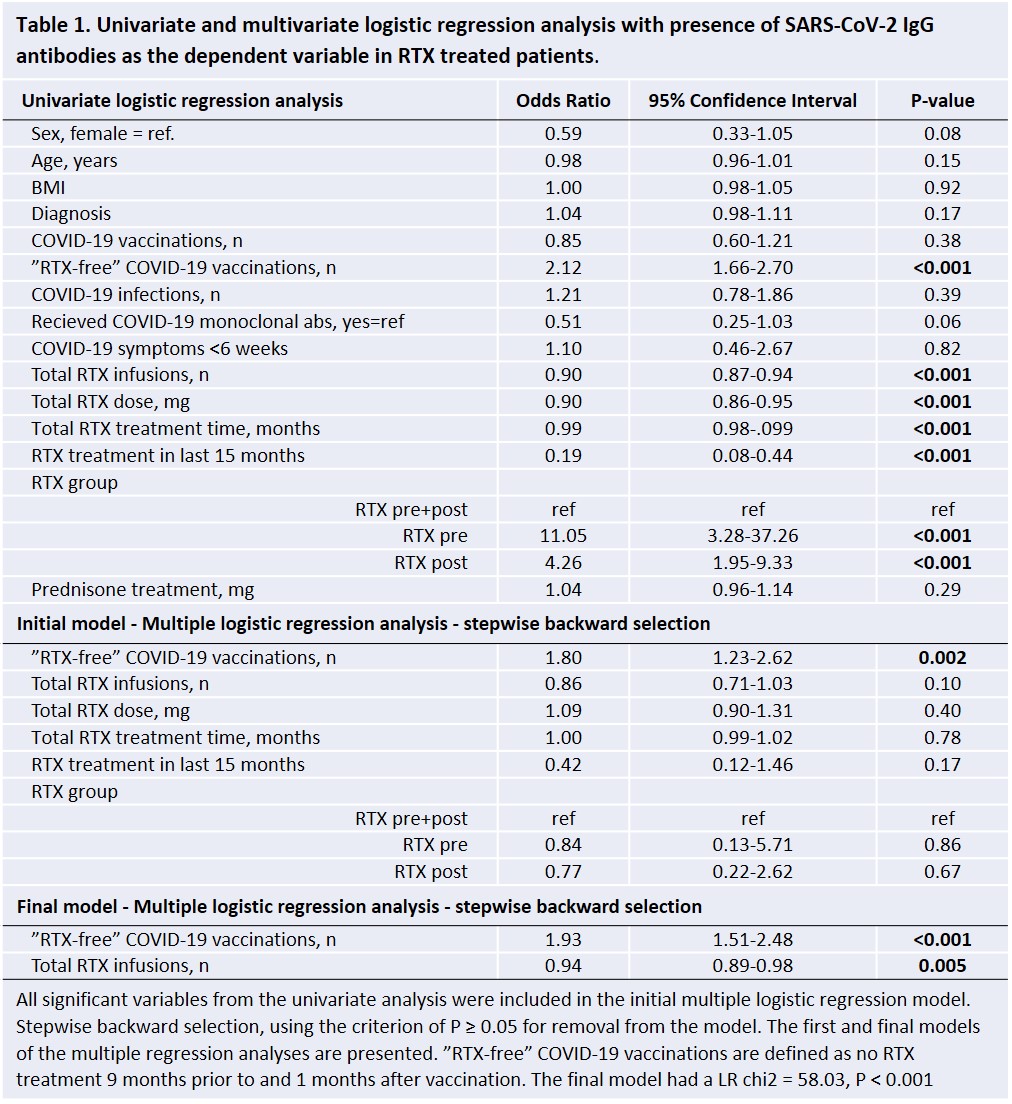
Univariate and multivariate logistic regression analysis were performed with seroconversion of SARS-CoV-2-Spike IgG as the dependent variable, Table 1. The final model had number of “RTX-free” vaccinations (OR 1.93) and number of RTX infusions (OR 0.94) as the significant predictors.
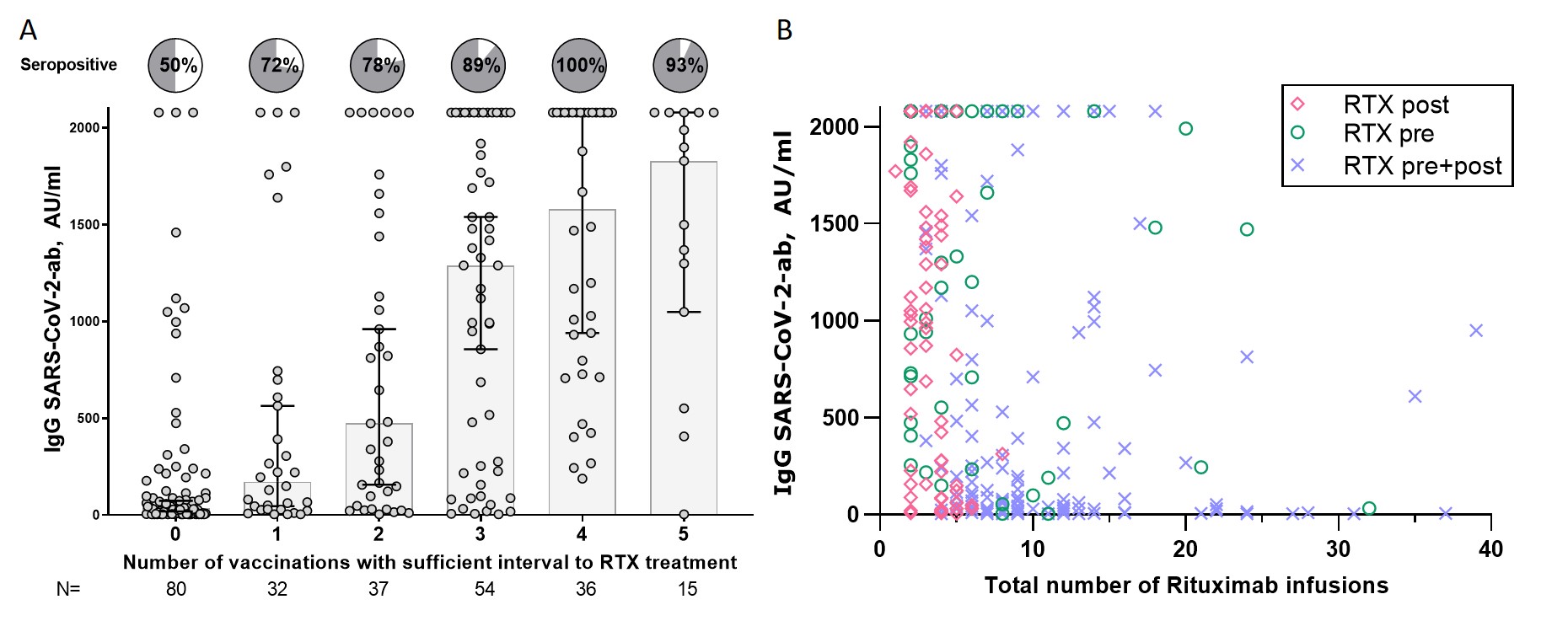
There was a significant dose-dependent correlation between “RTX-free” vaccinations with both antibody concentrations and seroconversion (Fig 2A, P< 0.001). The patients with zero “RTX-free” vaccinations had a seroconversion rate of 50%, compared to 89% for patients with 3 “RTX-free” vaccinations and40-fold higher median ab levels.
Conclusion: Our study demonstrates that COVID-19 vaccination administered before initiating RTX will induce a superior serological response compared to vaccination after RTX treatment. This supports the continuous use of RTX in rheumatic diseases with an emphasis on administering vaccinations before initiating RTX. Additionally, our findings highlight the importance of timing vaccinations for individuals undergoing continuous RTX treatment. The concept of “RTX-free” vaccinations with a minimum gap of 9 months since last RTX treatment may be useful in guiding vaccination strategies.
To cite this abstract in AMA style:
Ammitzbøll C, Thomsen M, Bartels L, Hermansen M, Hänel M, Klose-Jensen R, Larsen M, Hansen C, Lauritsen M, Mikkelsen S, Mistegaard C, Nielsen M, Naeser E, Olesen J, Garred P, Erikstrup C, Hauge E, Troldborg A. Superior SARS-CoV-2 Antibody Response Achieved in Rituximab-treated Patients When Vaccinated Against COVID-19 Before Compared to After Rituximab Initiation – Guidance for Future Vaccination Strategies [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/superior-sars-cov-2-antibody-response-achieved-in-rituximab-treated-patients-when-vaccinated-against-covid-19-before-compared-to-after-rituximab-initiation-guidance-for-future-vaccination-st/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/superior-sars-cov-2-antibody-response-achieved-in-rituximab-treated-patients-when-vaccinated-against-covid-19-before-compared-to-after-rituximab-initiation-guidance-for-future-vaccination-st/