Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: This study aims to compare the drug survival, effectiveness, and safety of anti-IL-17A and anti-TNF drugs in patients with Ankylosing Spondylitis (AS) following the failure of first-line anti-TNF therapy. The analysis utilizes real-life data from the Czech biologics registry ATTRA, which is a prospective observational cohort study capturing over 90% of AxSpA patients treated with bDMARDs and tsDMARDs in the Czech Republic. Anti-IL-17A bDMARDs have been reimbursed for AS in the Czech Republic since Jan 2016.
Methods: AS patients who initiated their second-line bDMARD between January 2016 and March 2023, with at least one year of follow-up, were included. Patients were divided into anti-IL-17A and anti-TNF cohorts based on their second bDMARD exposure. Effectiveness was assessed every 3-6 months using ASDAS (primary outcome), and secondary outcomes included pain measured on VAS, BASDAI, and CRP levels. Propensity score (PS) matching was applied to account for baseline differences at the time of switching treatments. The PS-matching used a logistic model with key baseline covariates (e.g. duration of first-line therapy, ASDAS, HAQ, CRP, swollen joint count, psoriasis) with a matching ratio of 1:3 and a caliper set to 0.1 using the nearest neighbor method. Drug survival was visualized with Kaplan-Meier curves. The incidence of adverse events (AEs) and serious adverse events (SAEs) per 1000 patient-years was also calculated.
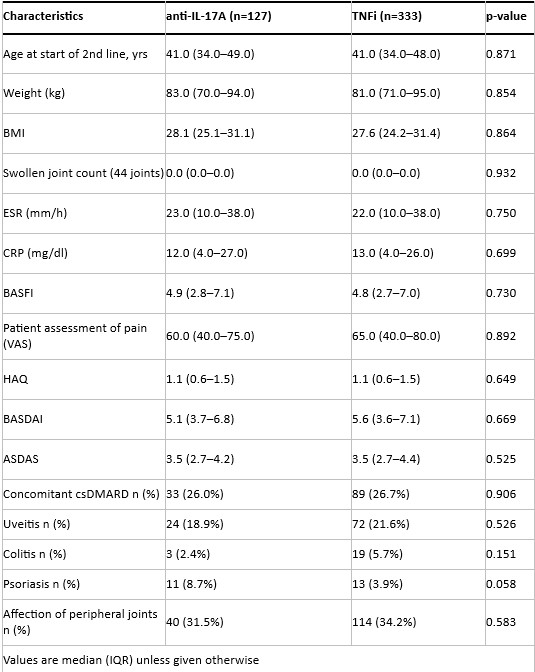
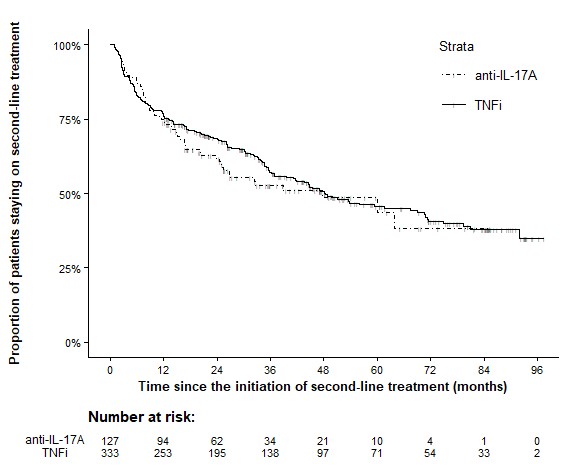
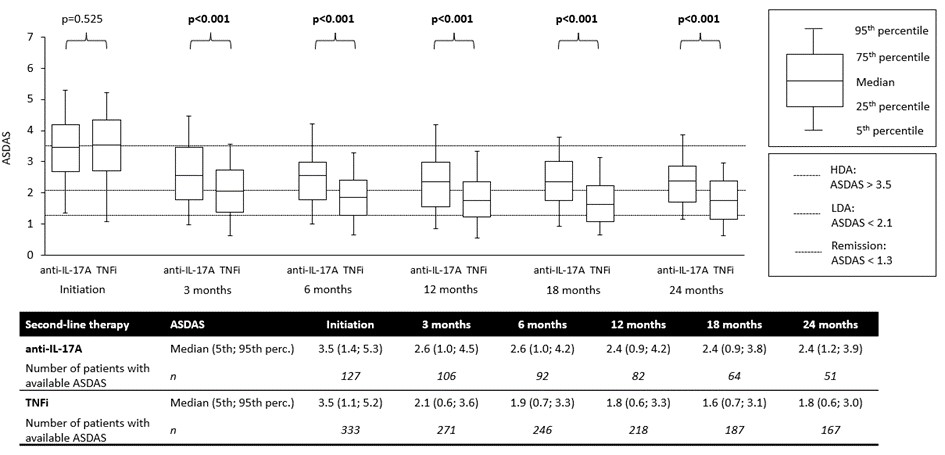
Results: A total of 1004 AS patients started second-line treatment with either anti-IL-17A (n=131) or anti-TNF (n=873) after failing first-line anti-TNF therapy within the specified period and had complete data for the variables of interest. PS-matching resulted in 127 patients on anti-IL-17A and 333 on anti-TNF. Baseline characteristics of the PS-matched cohorts are presented in Table 1. Drug retention rates were similar for both drug classes (Figure 1). In patients remaining on second-line therapy, ASDAS scores (Figure 2), VAS-pain, BASDAI, and CRP levels (data not shown) were significantly lower from month 3 to month 18 after starting anti-TNF therapy compared to anti-IL-17A therapy. The incidence of AEs and SAEs was numerically lower in the anti-IL-17A group than in the anti-TNF group (85 vs 148 AEs and 11 vs 21 SAEs per 1000 patient-years, resp.).
Conclusion: In patients AS who failed first-line anti-TNF therapy, switching to another anti-TNF drug resulted in similar drug survival but significantly better effectiveness (as measured by ASDAS, CRP, BASDAI, and VAS for pain) but numerically lower safety (incidence of AEs and SAEs) compared to switching to an IL-17A inhibitor.
Acknowledgements: Supported by project 00023728 of Ministry of Health, CZ.
To cite this abstract in AMA style:
Závada J, Baranová J, Pavelka K, Vencovský J, Horak P, Kusalová K, Šenolt L. Superior Effectiveness and Comparable Drug Retention of Anti-TNF Therapy versus Anti-IL-17A in Ankylosing Spondylitis After First Anti-TNF Failure: A Propensity Score-Matched Analysis from the Czech ATTRA Registry [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/superior-effectiveness-and-comparable-drug-retention-of-anti-tnf-therapy-versus-anti-il-17a-in-ankylosing-spondylitis-after-first-anti-tnf-failure-a-propensity-score-matched-analysis-from-the-czech-a/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/superior-effectiveness-and-comparable-drug-retention-of-anti-tnf-therapy-versus-anti-il-17a-in-ankylosing-spondylitis-after-first-anti-tnf-failure-a-propensity-score-matched-analysis-from-the-czech-a/