Session Information
Date: Sunday, October 21, 2018
Title: Rheumatoid Arthritis – Treatments Poster I: Strategy and Epidemiology
Session Type: ACR Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: In France, tocilizumab (TCZ) subcutaneous (sc) 1st prescription and yearly renewal are restricted to hospital. Monitoring may be done by both office-based or hospital rheumatologists. The objective is to describe 12-month drug retention rate, efficacy and tolerance of TCZ sc in real life in rheumatoid arthritis (RA) patients (pts).
Methods: Prospective, multicentre, observational 18-month study including pts with moderate/severe active RA requiring TCZ sc as prescribed in real life. Primary endpoint: drug retention rate of TCZ sc at 12 months in pts followed by hospital- and office-based rheumatologists. Secondary endpoints: pts characteristics, concomitant treatments, adherence using the Compliance Questionnaire for Rheumatology (CQR5), efficacy and safety of TCZ sc. Statistical analysis: pts with ≥1 TCZ injection were analyzed for safety. Pts fulfilling inclusion and non-inclusion criteria were analyzed for efficacy.
Results: 291 pts were recruited, 288 were analysed for safety and 281 for efficacy. At baseline: mean age 56.2±12.5 years, females: 74.4%, at least 1 co-morbidity: 71%, mean RA duration 9.5±9.0 years, RF/ACPA +: 83.5%, erosive RA: 61.2%, mean DAS28ESR 4.76±1.22. Past RA treatments included DMARDs in 94.3%, mainly MTX (90.4%), and biologics in 63.3%. TCZ Mono (i.e. without csDMARD) was initiated in 42% and TCZ Combo in 57% of the pts, 84.9% of the latest received MTX (mean dose 17.2±4.4 mg/w). 151 pts completed M12, 26 pts had no M12 visit collected yet. 104 (37.1%) pts withdrew: AE 19.4%, lack of efficacy 11.1%.
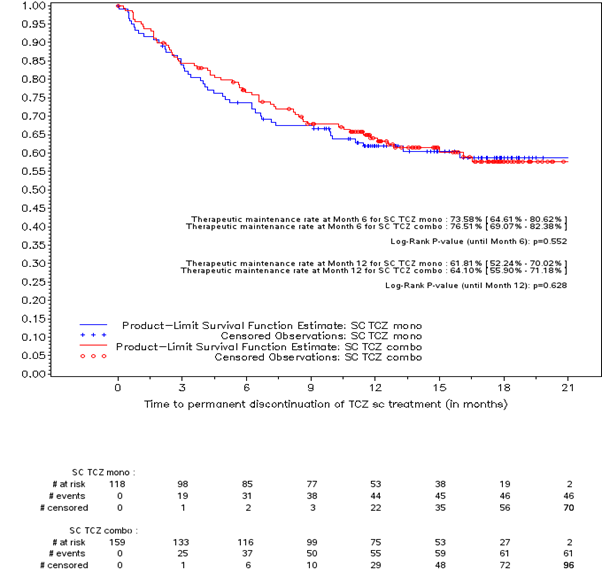
At M12, drug retention rate was 63.3% in all pts, 61.8% in Mono, 64.1% in Combo (Fig.1). 2 Mono pts had an ongoing csDMARD, 6 Combo pts had no csDMARD. Total number of TCZ injections was 33.3±23.3 done by pt himself in 53.9%, at home in 92.7%. Adherence to TCZ sc was high in 85.4% of the 82 pts who fulfilled the CQR5. 47.3% used prednisone at 13.1±12.8 mg/day in the Mono and 8.4±4.7 in the Combo group.
At M12, 89% of the pts were followed at hospital, 11% by mixed or office-based rheumatologists. Mean DAS28ESR in all, Mono and Combo were 1.89±1.08, 1.67±1.03, 2.05±1.11 respectively. DAS28 remission and LDA were respectively 71.8% in all, 77.1% in Mono, 67.2% in Combo and 16.4% in all, 14.6% in Mono, 18.0% in Combo. No new safety signal was reported. 218 (75.7%) pts had at least one adverse event (AE), 41 (14.2%) had at least one serious AE including 7 serious infections.
Conclusion: In this 12-month interim analysis, drug retention rate was 63.3% in patients receiving TCZ sc in real life, with no difference between Mono and Combo groups. DAS28 remission/LDA was 88.2% in all patients, 91.7% in Mono and 85.2% in Combo. No new safety signal occurred. Final analysis will include remaining missing data.
Fig. 1. Kaplan-Meier curve of the drug retention rate in Mono and Combo groups – Efficacy population
To cite this abstract in AMA style:
Hilliquin P, Barnetche T, Baudens G, Niarra R, Idier I, Saraux A. Subcutaneous Tocilizumab in Monotherapy or in Combination with DMARD in Patients with Moderate to Severe Active Rheumatoid Arthritis: Observational Study to Describe Real-World Drug Retention Rate at 12 Months, Interim Analysis [abstract]. Arthritis Rheumatol. 2018; 70 (suppl 9). https://acrabstracts.org/abstract/subcutaneous-tocilizumab-in-monotherapy-or-in-combination-with-dmard-in-patients-with-moderate-to-severe-active-rheumatoid-arthritis-observational-study-to-describe-real-world-drug-retention-rat/. Accessed .« Back to 2018 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/subcutaneous-tocilizumab-in-monotherapy-or-in-combination-with-dmard-in-patients-with-moderate-to-severe-active-rheumatoid-arthritis-observational-study-to-describe-real-world-drug-retention-rat/