Session Information
Date: Tuesday, November 12, 2019
Title: 5T090: Osteoarthritis – Clinical II: Novel Therapies (2756–2761)
Session Type: ACR Abstract Session
Session Time: 2:30PM-4:00PM
Background/Purpose: Tanezumab, a monoclonal antibody inhibiting nerve growth factor (NGF), is under investigation for the treatment of chronic pain conditions. In prior osteoarthritis (OA) studies, intravenous tanezumab was effective and generally well tolerated, and the risk of increased joint damage required further assessment. This study was conducted to evaluate the efficacy and safety of subcutaneous (SC) tanezumab vs NSAID in patients (pts) with OA; findings on the long-term risk of joint safety events are presented here.
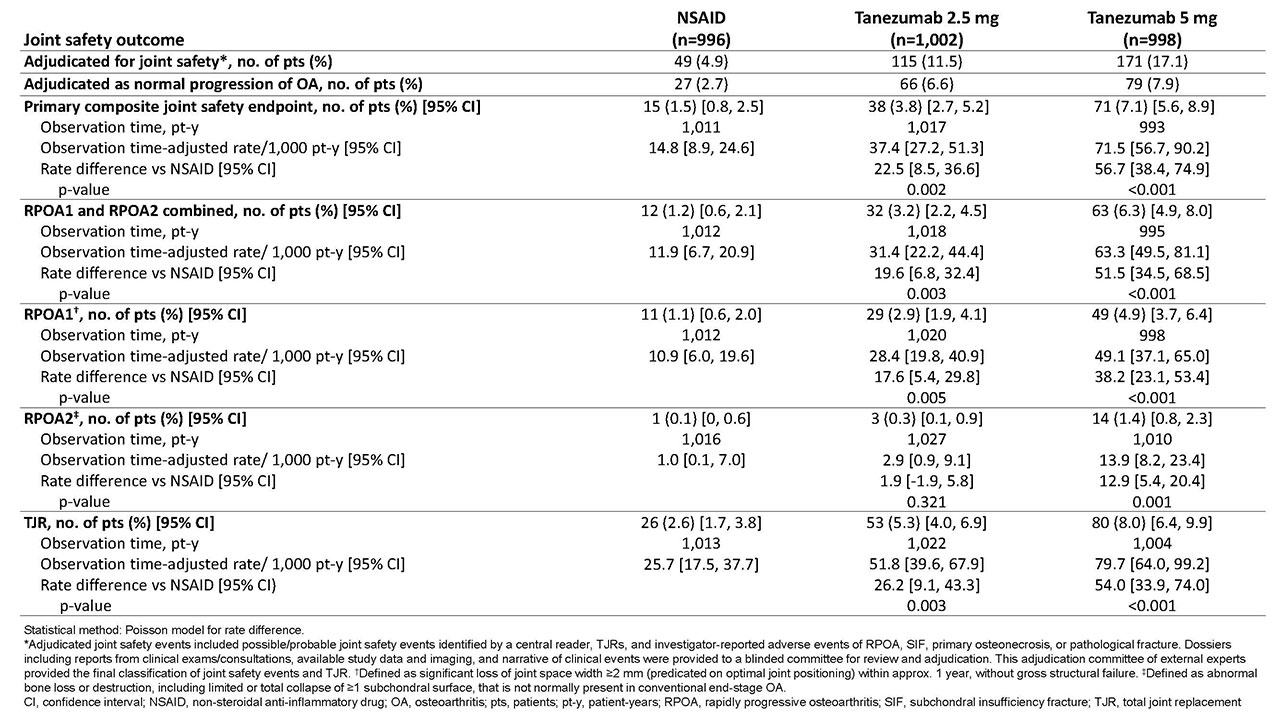
Methods: Eligible pts had OA of the hip or knee based on ACR criteria with x-ray confirmation; baseline (BL) WOMAC Pain and Physical Function scores of ≥5 (11-pt numeric rating scale [0-10]); BL Patient’s Global Assessment of OA (PGA-OA) of “fair,” “poor,” or “very poor”; history of inadequate pain relief with acetaminophen; inadequate pain relief with/intolerance to tramadol or an opioid; or unwillingness to take an opioid. Pts were on a stable dose of oral NSAID before study entry and during screening. Pts were randomized to tanezumab (2.5 mg or 5 mg SC q 8 wk) or NSAID (naproxen 500 mg, celecoxib 100 mg, or diclofenac ER 75 mg orally bid) for 56 wk. Radiographic/magnetic resonance imaging was performed at screening, wk 24 and 56 (treatment period), and wk 80 (safety period). Pts meeting pre-specified criteria for increased severe or persistent joint pain had additional follow-up imaging. All images were evaluated by central reader; joint safety events and total joint replacements (TJR) were adjudicated by blinded committee. Observation time-adjusted rates/1,000 pt-y of adjudicated rapidly progressive OA type 1 or 2 (RPOA1 or 2), primary osteonecrosis, subchondral insufficiency fracture (SIF), and pathologic fracture over 80 wk were analyzed (combined as primary composite joint safety endpoint and individually), as was the TJR rate.
Results: A total of 2,996 pts were randomized, received ≥1 dose of study drug, and were analyzed for joint safety events. During 80 wk of observation, the time-adjusted rate/1,000 pt-y for the primary composite joint safety endpoint was higher with tanezumab 2.5 mg (37.4 [p=0.002]) and tanezumab 5 mg (71.5 [p< 0.001]) than NSAID (14.8) (Table). The majority of joint safety events adjudicated as RPOA were RPOA1 (89 of 107 [83.2%]). Rates of RPOA1 were higher with tanezumab 2.5 mg (28.4 [p=0.005]) and tanezumab 5 mg (49.1 [p< 0.001]) than NSAID (10.9), as were rates of RPOA2 (2.9 [p=0.321] and 13.9 [p=0.001] vs 1.0, respectively). In the tanezumab 2.5 and 5 mg groups, SIF rates were 5.8 (p=0.539) and 6.9 (p=0.364) vs 3.9 in the NSAID group. Primary osteonecrosis occurred in 1 pt in the tanezumab 5-mg group; no pathological fractures were observed. TJR rates were also higher with tanezumab 2.5 mg (51.8 [p=0.003]) and 5 mg (79.7 [p< 0.001]) than NSAID (25.7). Of 159 pts with TJR, 28 (17.6%) had an adjudicated joint safety outcome; in these 28 pts, the event was RPOA1 in 42.9%; RPOA2, 39.3%; primary osteonecrosis, 3.6%; and SIF, 14.3%.
Conclusion: In this study, adjudicated joint safety endpoints, particularly RPOA1, and TJR were significantly more common with tanezumab than NSAID. Additional research is ongoing to explore potential reasons for this imbalance and dose-response relationships.
To cite this abstract in AMA style:
Hochberg M, Carrino J, Schnitzer T, Guermazi A, Walsh D, White A, Nakajo S, Fountaine R, Hickman A, Pixton G, Viktrup L, Brown M, West C, Verburg K. Subcutaneous Tanezumab versus NSAID for the Treatment of Osteoarthritis: Joint Safety Events in a Randomized, Double-Blind, Active-Controlled, 80-Week, Phase-3 Study [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/subcutaneous-tanezumab-versus-nsaid-for-the-treatment-of-osteoarthritis-joint-safety-events-in-a-randomized-double-blind-active-controlled-80-week-phase-3-study/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/subcutaneous-tanezumab-versus-nsaid-for-the-treatment-of-osteoarthritis-joint-safety-events-in-a-randomized-double-blind-active-controlled-80-week-phase-3-study/