Session Information
Date: Friday, November 6, 2020
Title: Spondyloarthritis Including Psoriatic Arthritis – Treatment Poster I
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Patients (pts) with ankylosing spondylitis (AS) report pain (70–80%), stiffness (20–40%), and fatigue (50–60%) as the most troubling symptoms. Early diagnosis and sustained improvement in these symptoms are essential for effective management of AS. Here, we report the effect of subcutaneous (s.c.) secukinumab (SEC) 150mg on these symptoms through 5 years (end-of-study) from MEASURE 2.
Methods: The MEASURE 2 study design has been reported previously. This post hoc analysis assessed the mean change from baseline to Week (Wk) 260 in total and nocturnal back pain scores (Visual analogue scale [0–100]), overall level of spinal pain (neck, back, or hip) from BASDAI score, and morning stiffness (overall level; mean of questions 5 and 6 of BASDAI score). Additionally, the SF-36 physical component summary (PCS) score, BASFI score, overall level of fatigue (BASDAI question 1), FACIT-Fatigue, ASDAS-CRP scores, and proportion of pts meeting minimal clinically important difference (MCID) criteria across multiple clinical domains were also assessed. Data are shown for pts originally randomized to SEC 150mg and placebo. Data are reported as observed for overall population and by prior tumor necrosis factor inhibitor (TNFi) therapy status (naïve vs inadequate response/intolerance [IR]).
Results: Baseline clinical characteristics were generally comparable across the treatment groups; SEC 150mg (N=72) and placebo (N=74). 73.6% (n=53) of pts originally randomized to SEC 150 mg completed 5 years of treatment. The most frequent reason of discontinuation was adverse events reported 9.7% (n=7) pts. Improvements were sustained through 5 years in pts treated with SEC 150mg in pain, morning stiffness, physical function (SF-36 PCS and BASFI), fatigue (overall level of fatigue and FACIT-Fatigue), and ASDAS-CRP (Table 1). 48.1% and 25.0% of SEC 150mg treated pts met MCID criteria for ASDAS-CRP Low Disease Activity and ASDAS-CRP Inactive Disease at 5 years, respectively (Table 2). Improvements were observed in both TNFi-naïve and -IR pts, with numerically higher improvement in TNFi-naïve pts.
Conclusion: Secukinumab 150mg provided clinically meaningful improvements in total back pain, nocturnal back pain, morning stiffness, fatigue and low disease activity through 5 years of treatment in pts with active AS.
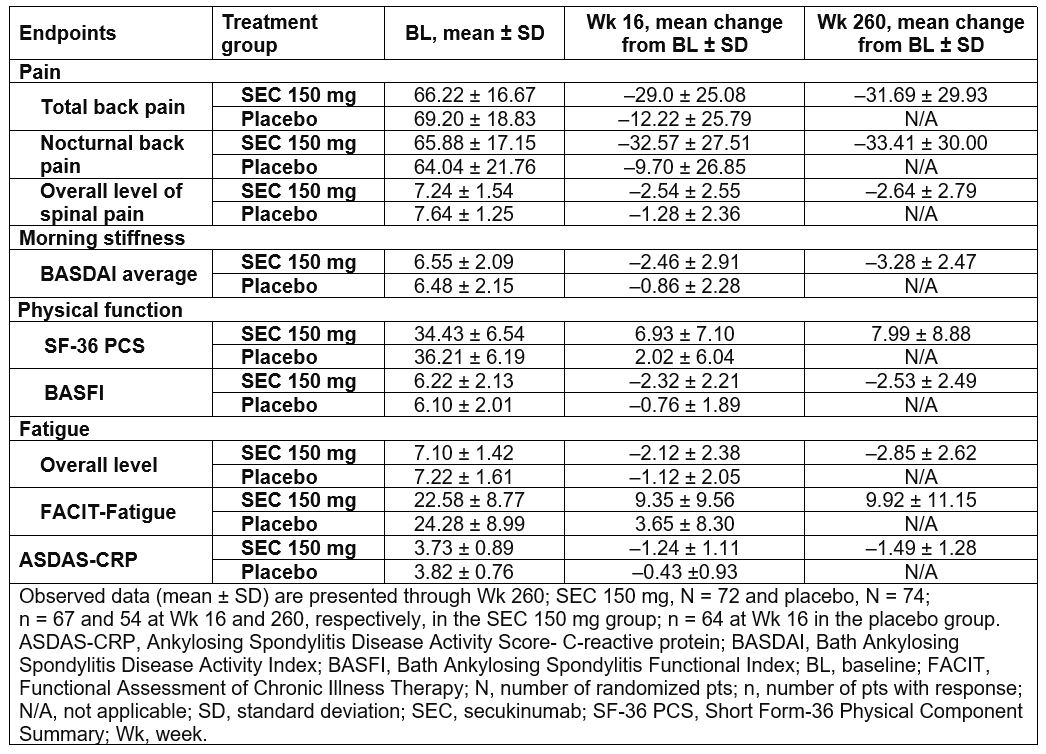 Table 1: Improvement in Pain, Morning Stiffness and Fatigue Scores Through Wk 260
Table 1: Improvement in Pain, Morning Stiffness and Fatigue Scores Through Wk 260
 Table 2: Proportion of Patients Meeting MCID Criteria Through Wk 260
Table 2: Proportion of Patients Meeting MCID Criteria Through Wk 260
To cite this abstract in AMA style:
Marzo-Ortega H, Miceli-Richard C, Gill S, Magrey M, Machado P, Shete A, Wang J, Rohrer S, Deodhar A. Subcutaneous Secukinumab 150 Mg Provides Sustained Relief in Total and Nocturnal Back Pain, Morning Stiffness, Fatigue, and Low Disease Activity in Patients with Active Ankylosing Spondylitis: End-of-study (5-year) Data from the MEASURE 2 Trial [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/subcutaneous-secukinumab-150-mg-provides-sustained-relief-in-total-and-nocturnal-back-pain-morning-stiffness-fatigue-and-low-disease-activity-in-patients-with-active-ankylosing-spondylitis-end-of/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/subcutaneous-secukinumab-150-mg-provides-sustained-relief-in-total-and-nocturnal-back-pain-morning-stiffness-fatigue-and-low-disease-activity-in-patients-with-active-ankylosing-spondylitis-end-of/
