Session Information
Date: Saturday, November 6, 2021
Title: Epidemiology & Public Health Poster I: COVID-19 & Vaccination (0084–0117)
Session Type: Poster Session A
Session Time: 8:30AM-10:30AM
Background/Purpose: Many uncertainties remain for IL-6 blockers on the management of COVID-19 such as the optimal time of intervention, the schedule of administration and predictors of response. To date, data on the use of subcutaneous sarilumab (SAR) is scarce and no randomized controlled trial (RCT) results are available. Therefore, we aimed at evaluating the effect of subcutaneous SAR vs standard of care (SOC) in hospitalized patients with moderate to severe COVID-19.
Methods: Single center open-label RCT. We included admitted adult patients with documented COVID-19 infection with pneumonia confirmed by chest imaging, laboratory evidence of inflammatory phenotype and no need for invasive ventilation. Patients were randomized from March 14th to October 30th 2020, to receive SAR or SOC in a 2:1 proportion. The experimental arm received a single 400 mg dose of SAR in two 200 mg subcutaneous injections added to SOC. The control arm received usual supportive care as per local protocols.
The primary endpoints included 30-day mortality, mean change in clinical status at day 7 on an ordinal scale ranging from death (category1) to discharged (category 7), and duration of hospitalization. The primary efficacy analysis was conducted on the intention to-treat population. Secondary outcomes included time to became afebrile for 48 hours without antipyretics, mean change in the mentioned ordinal scale at day 14, progression to non- invasive mechanical ventilation (NIMV) and invasive mechanical ventilation (IMV), time to oxygen supply independency, and adverse events (AE). To estimate the intervention effect size, hazard ratios (HR) were estimated when feasible.
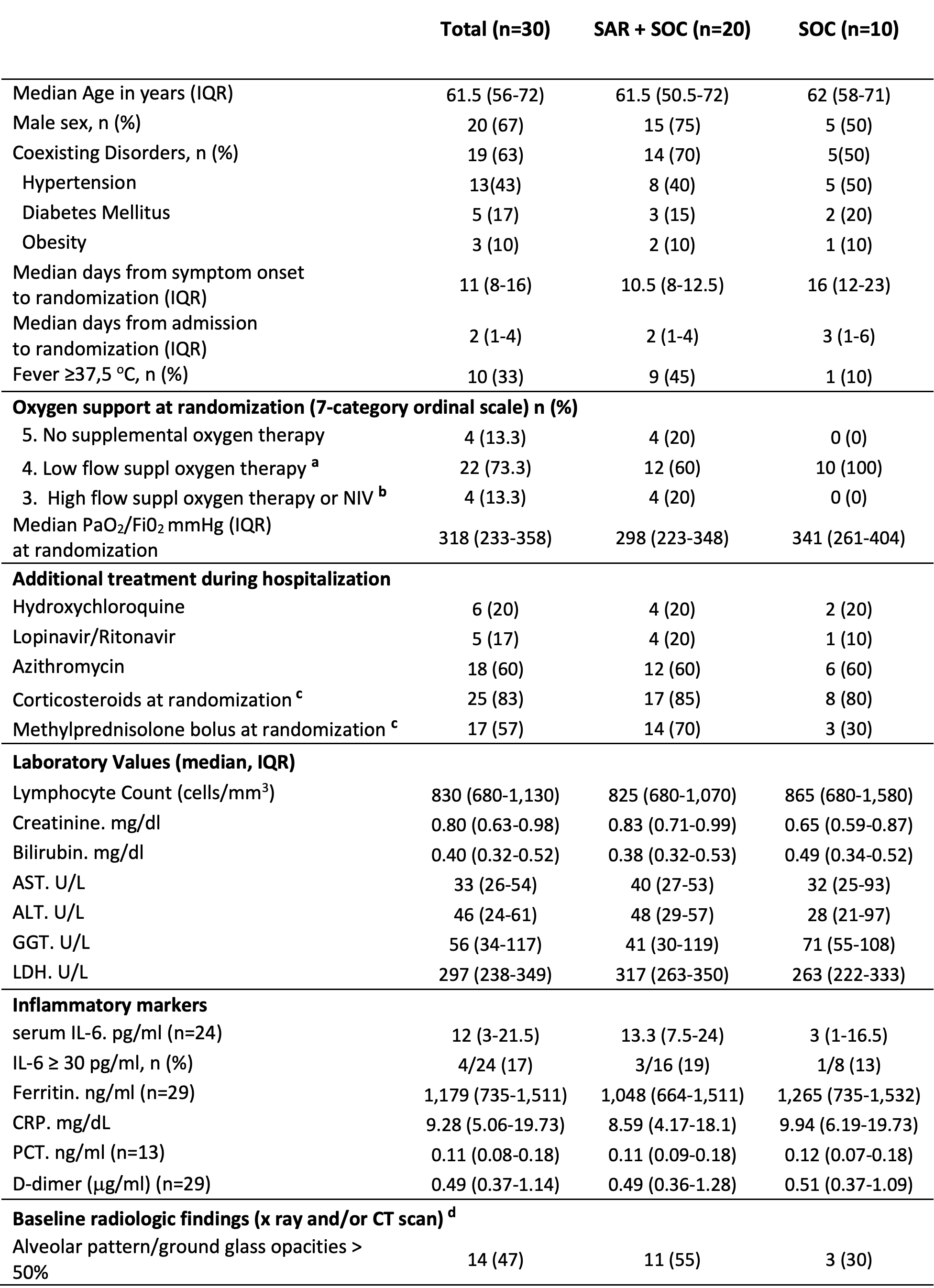
Results: Thirty out of sixty-five screened patients underwent randomization: 20 to SAR and 10 to SOC. Most patients were male (20/30, 67%) with a median (interquartile range, IQR) age of 61.5 (56-72) (Table 1).
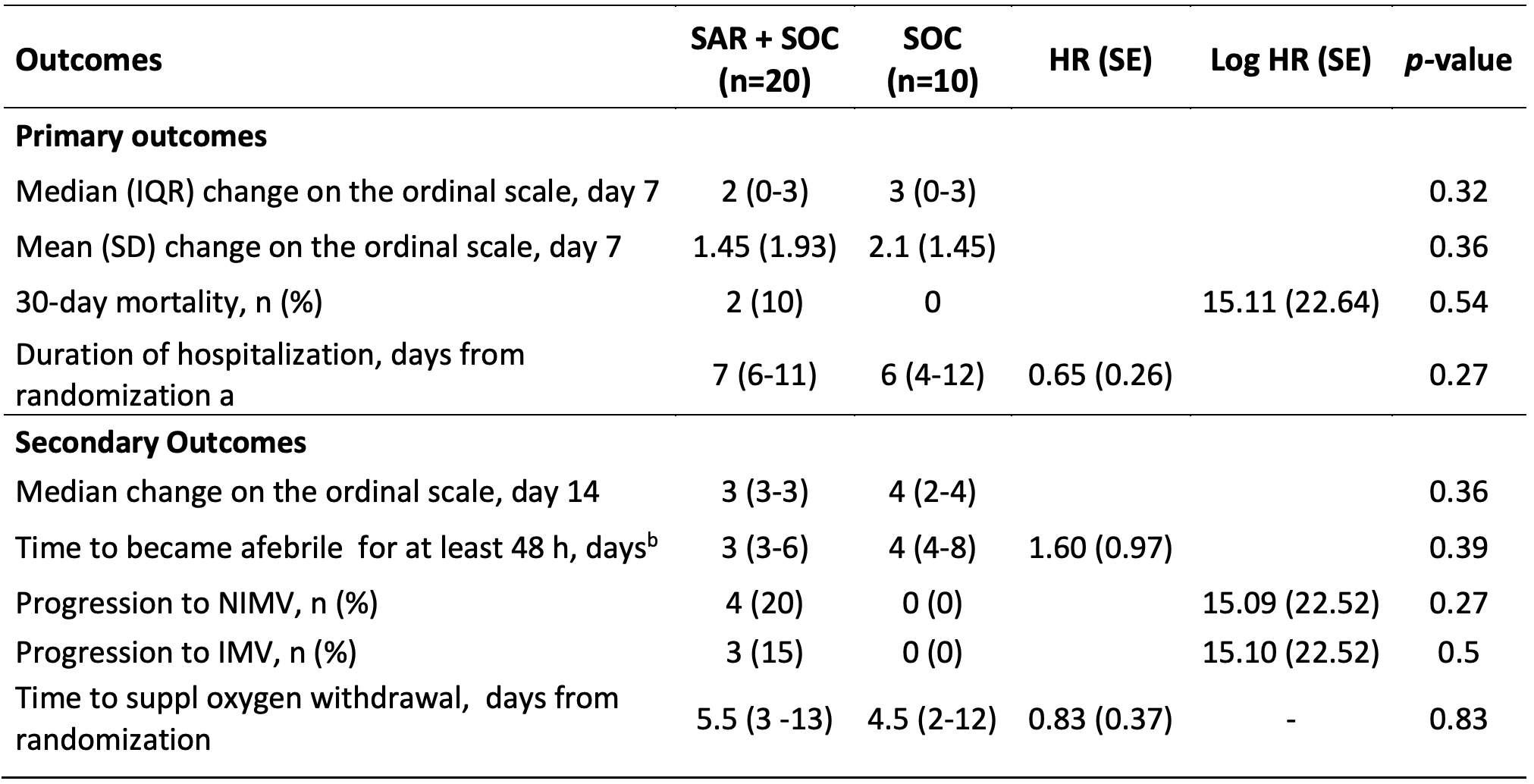
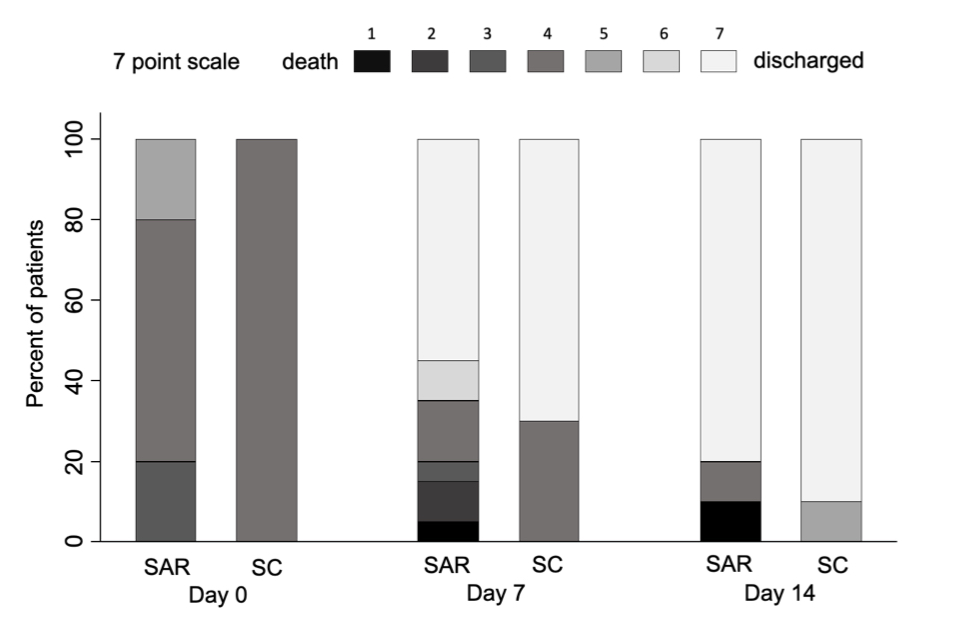
At day 30, 2/20 patients died in the SAR and none in the SOC arm (Log HR 15.11, SD 22.64, p 0.54). At day 7, no significant differences were seen in the median change on a 7-category ordinal scale (2 [0-3] vs 3 [0-3], p 0.32) (Figure 1). The median days to discharge were similar (7 [6-11] vs 6 [4-12]; HR 0.65, SD 0.26; p 0.27). No significant differences were seen for any of the secondary outcomes. In the SAR arm, 4/20 (20%) and 3/20 (15%) patients required NIMV and IMV respectively vs none in the SOC (Table 2). Regarding safety, the rate of AE of special interest were similar in the SAR (50%) and SOC (40%) arms.
Conclusion: In our study, subcutaneous SAR added to SOC showed no additional benefit in 30-day mortality, clinical status at day 7, or hospital stay.
AST: Aspartate amino-transferase; ALT: Alanine amino- transferase; COPD: Chronic obstructive pulmonary disease; CRP: C-reactive protein; GGT: Gamma-glutamyl transferase; IL-6: Interleukin-6; LDH: Lactate Dehydrogenase; NIV: noninvasive ventilation; PaO2/Fi02: partial pressure of arterial oxygen/fraction of inspired oxygen; PCT: Procalcitonin. a) O2 flow ≤ 15l/min e.g. by face mask, nasal cannula (NC); b) O2 flow >15l/min, e.g. by face mask, ‘High Flow’ devices (e.g. HFNC), CPAP or NIV including BiPAP and other devices; c) Corticosteroids: 30 mg Prednisone/d or equivalent; intravenous bolus of 6-Metilprednisolone 120_125 mg/d, except 1 patient 80 mg/d; d) All radiologic exams were assessed and reported by radiologists with pneumological expertise
IMV: invasive mechanical ventilation; NIMV: Noninvasive mechanical ventilation; SD: standard deviation; SOC: Standard of care. a) Accounting for survival status by treating patients who died as having a 30-day hospital stay; b) Eleven patients in the SAR arm and 5 in the SOC arm were febrile at randomization
To cite this abstract in AMA style:
Rodriguez-García S, Gonzalez-Alvaro I, Abad-Santos F, Bautista-Hernández A, García-Fraile L, Baldivieso-Achá J, Sanz-Sanz J, Garcia de Vicuña R. Subcutaneous Sarilumab in Hospitalized Patients with Moderate-severe COVID-19 Infection Compared to the Standard Care: An Open-label Randomized Clinical Trial [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/subcutaneous-sarilumab-in-hospitalized-patients-with-moderate-severe-covid-19-infection-compared-to-the-standard-care-an-open-label-randomized-clinical-trial/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/subcutaneous-sarilumab-in-hospitalized-patients-with-moderate-severe-covid-19-infection-compared-to-the-standard-care-an-open-label-randomized-clinical-trial/