Session Information
Date: Tuesday, November 10, 2015
Title: Rheumatoid Arthritis - Small Molecules, Biologics and Gene Therapy Poster III
Session Type: ACR Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose:
The CAnadian Methotrexate
and Etanercept Outcome (CAMEO) study, an open-label trial in
patients with active rheumatoid arthritis (RA), revealed that patients
achieving low disease activity (LDA) after 6 months of etanercept (ETN) plus
methotrexate (MTX) therapy had similar clinical outcomes at 12 months if they
continued ETN+MTX, or switched to ETN alone, at month 6
(M6). Conversely, those who did not achieve LDA at M6 had a reduced response
after switching to ETN monotherapy. This analysis assessed the
different discontinuation rates between treatment arms in the CAMEO trial by
examining study completion and ETN retention over 25 months, and the association
between ETN discontinuation and disease activity.
Methods:
Anti-TNF naïve patients with
active RA (≥ 3 swollen joints, disease activity score [DAS28] ≥
3.2), despite MTX treatment (≥ 15 mg/week or 10 mg/week if intolerant)
for > 12 weeks, were enrolled. After 6 months of ETN (50 mg/week
subcutaneously) + MTX treatment, patients were randomized (1:1) to ETN+MTX or ETN
alone for another 18 months. ETN retention was assessed by physician
prescribing information at the end of study. Cox regression analysis determined
associations of study completion and ETN retention with DAS28 score at M6
(continuous variable; not dichotomized to LDA and moderate/high disease
activity), reimbursement type, and demographics.
Results:
A total of 258 patients
enrolled (76% female, mean age 54.7 ± 12.5 years, disease duration 8.9 ± 8.4
years, baseline DAS28 5.4 ± 1.1) and 205 (79%) were randomized at M6 (98 ETN, 107
ETN+MTX). Of the 205 patients randomized, 50 (51.0%) and 75 (70.1%) in the ETN
and ETN+MTX groups completed the study, respectively, with the remainder
discontinuing due to adverse events, lack of efficacy, or other reasons. Patients
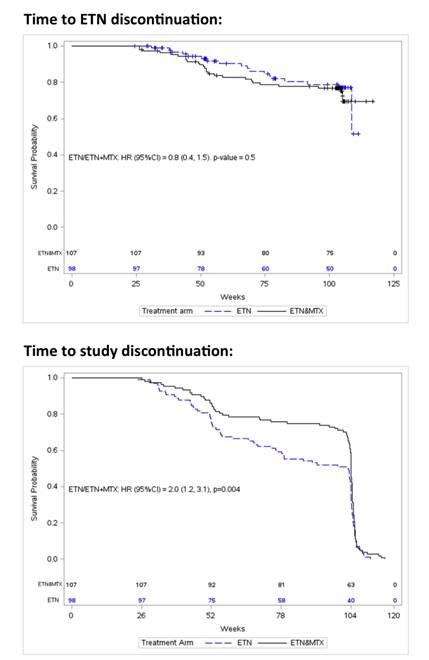
on ETN alone were twice as likely to discontinue the study as those on ETN+MTX
(HR [95% CI] 2.0 [1.2, 3.1], p=0.004), and with increasing DAS28 score at M6,
the chance of study discontinuation increased by 20% in both treatment arms (HR
[95% CI] 1.2 [1.1, 1.5], p=0.005). Of the 205 patients randomized, 80 (81.6%)
and 80 (74.8%) in the ETN and ETN+MTX groups, respectively, continued ETN at the
end of the study but some randomized to ETN alone restarted MTX. ETN retention
did not differ between treatment arms (HR [95% CI] 0.8 [0.4, 1.5], p=0.5), but
higher DAS28 at M6, increased the chance of discontinuing ETN in both groups (HR
[95% CI] 1.4 [1.1, 1.8], p=0.0017). Reimbursement and demographics were not
associated with study and/or ETN discontinuation.
Conclusion:
Lower disease activity at M6 increased
the chance of study completion and ongoing ETN treatment. Patients on ETN+MTX
vs. ETN alone, and those achieving LDA at M6, regardless of treatment group,
were more likely to complete the study. Both treatment arms had high ETN
retention rates at the end of the study, with those reaching LDA at M6 being
more likely to remain on ETN.
To cite this abstract in AMA style:
Pope JE, Keystone EC, Thorne JC, Poulin-Costello M, Phan-Chronis K, Haraoui B. Study Completion and Etanercept Retention in Patients with Rheumatoid Arthritis Treated with Etanercept Monotherapy Versus Etanercept and Methotrexate Combination Therapy [abstract]. Arthritis Rheumatol. 2015; 67 (suppl 10). https://acrabstracts.org/abstract/study-completion-and-etanercept-retention-in-patients-with-rheumatoid-arthritis-treated-with-etanercept-monotherapy-versus-etanercept-and-methotrexate-combination-therapy/. Accessed .« Back to 2015 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/study-completion-and-etanercept-retention-in-patients-with-rheumatoid-arthritis-treated-with-etanercept-monotherapy-versus-etanercept-and-methotrexate-combination-therapy/