Session Information
Date: Sunday, October 21, 2018
Title: Pediatric Rheumatology – Clinical Poster I: Lupus, Sjögren’s Disease, and Myositis
Session Type: ACR Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose:
The use of physical activity monitors (PAM), which objectively quantify free-living movement, may enhance assessment of disease activity in juvenile myositis (JM) clinical trials and complement traditional core set measures (CSM) of disease activity. We examined the validity, reliability and responsiveness of the average daily step counts (ADSC) obtained from a commercially available PAM, Fitbit¨ One, as outcome measures in patients with JM.
Methods:
JM patients age 3-17 were enrolled from rheumatology clinic. The following evaluations were performed at baseline, 1, 3 and 6 months: a) CSMs including Manual Muscle Testing (MMT), Childhood Health Assessment Questionnaire (CHAQ), MD global disease activity (MD-global), Extra-muscular global disease activity (Ex-Mus global), muscle enzyme, patient/parent global disease activity (pt-global), Childhood Myositis Assessment Scale (CMAS), Disease Activity Score (DAS), b) Patient-Reported Outcome (PRO) Measurement Information System Mobility Short Form (PROMIS-SF), and c) office functional tests: Sit-to-Stand (STS), Timed Up and Go (TUG) and Six Minute Walk Distance (6MWD). Fitbit¨ One was worn for 7 consecutive days monthly for 6 months, and PAM measures, including ADSC were assessed. Pearson Correlation assessed the relationship between Fitbit¨ ADSC and current CSMs, PROs and functional tests. Reliability and responsiveness of PAM measures were determined.
Results:
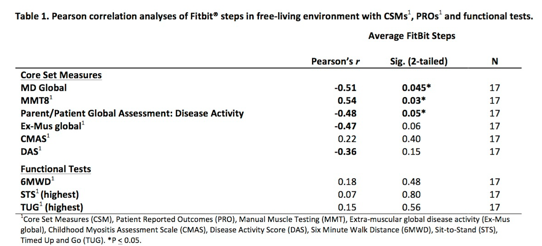
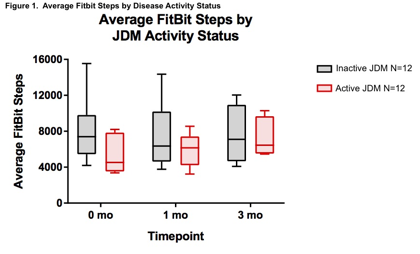
A total of 17 JM participants have been enrolled, including 5 active and 12 stable, 76.5% female, 94% White with mean (SD) age of 11.5 years (3.4). As the study is ongoing, this data represents baseline, 1 and 3-month time points. Fitbit¨ ADSC showed moderate to strong correlation at baseline with key CSMs including MD global, MMT, pt-global, Ex-Mus global, but no to weak correlation with CMAS and functional tests (Table 1). Patients with stable disease at baseline and follow-up (1 and 3 months) showed no significant change in Fitbit¨ ADSC over time demonstrating strong test-retest reliability, whereas patients with active disease demonstrated Fitbit¨ ADSC increase longitudinally associated with clinical improvement (Figure 1).
Conclusion:
Fitbit¨ ADSC had moderate to strong correlation with key JM CSMs except CMAS and functional test. ADSC also demonstrate preliminary reliability and responsiveness. Continued analysis of longitudinal data will help to determine the utility of a commercially available PAM as an outcome measure in JDM.
To cite this abstract in AMA style:
Brunner E, Tasan L, Torok KS, Rockette-Wagner B, Zigler CK, Schollaert-Fitch K, Koontz D, Oddis CV, Aggarwal R. Stepping It up: The Use of Physical Activity Monitors As an Outcome Measure in Juvenile Myositis [abstract]. Arthritis Rheumatol. 2018; 70 (suppl 9). https://acrabstracts.org/abstract/stepping-it-up-the-use-of-physical-activity-monitors-as-an-outcome-measure-in-juvenile-myositis/. Accessed .« Back to 2018 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/stepping-it-up-the-use-of-physical-activity-monitors-as-an-outcome-measure-in-juvenile-myositis/