Session Information
Date: Sunday, November 13, 2016
Title: Osteoarthritis – Clinical Aspects I: Epidemiology and Progression
Session Type: ACR Concurrent Abstract Session
Session Time: 4:30PM-6:00PM
Background/Purpose: Development of disease-modifying osteoarthritis drugs (DMOADs) for knee osteoarthritis (OA) has been challenging, partially owing to lack of prognostic biomarkers. Our objective was to identify baseline (BL) biomarkers of rapid OA progression and estimate the potential value and cost of using biomarkers in a knee OA trial.
Methods: The Foundation for the National Institute of Health/Osteoarthritis Initiative (fNIH/OAI) Biomarker Consortium included 600 patients (pts) with knee OA. Pts with nonprogression (n=200), pain progression (n=100), x-ray progression (n=100), and pain/x-ray progression combined (n=200) had knee x-rays, MRIs, and blood and urine biomarkers of bone and cartilage damage. BL biomarkers associated with progression were identified using receiver operator characteristics and multivariate models (Random Forest, Neuro-Network, and Monte Carlo empowered LASSO). To identify subgroups, Patient Rule Induction Method and the Adaptive Index Model were used. To evaluate the value of implementing biomarkers in a trial, we simulated biomarker data based on the OAI population, from which fNIH samples were selected. We estimated potential benefit (reduction in sample size) and cost (screen failure rate) of the biomarker strategy for conducting a knee OA trial.
Results: BL urine CTX-II was the best soluble biomarker predicting OA progression in 12 of 25 multivariate models tested, although predictive power was low (AUROC <60%; Table 1). Use of urine CTX-II during the screening phase of a DMOAD trial enriched the number of pts with OA progression by 13% and reduced sample size by 45%; screen failure rate was 83% (Fig; Table 2). The number of medial tibiofemoral subregions affected by MRI bone marrow lesion (BML) was one of the best imaging predictors of OA progression. BL MRI BMLs enriched the number of pts with OA progression by 20% and reduced sample size by 62%; screen failure rate was 78% (Fig; Table 2).
Conclusion: A novel approach combining statistical analysis of fNIH/OAI data with clinical simulations indicated that stratification using urine CTX-II and MRI BML would reduce the sample size needed for a DMOAD clinical trial. However, use of CTX-II and MRI BML could increase the screen failure rate and preclude their implementation as stratification biomarkers in clinical trials. 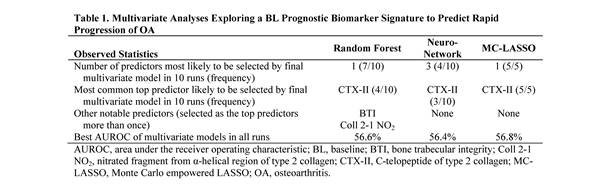


To cite this abstract in AMA style:
Feng S, Liu Z, Hong F, Medema J, Kamath R, Levesque MC. Statistical Simulation Using Data from the Foundation for the National Institute of Health/Osteoarthritis Initiative Biomarkers Consortium to Evaluate the Clinical Utility of Prognostic Knee Osteoarthritis Biomarkers in Designing a Knee Osteoarthritis Clinical Trial [abstract]. Arthritis Rheumatol. 2016; 68 (suppl 10). https://acrabstracts.org/abstract/statistical-simulation-using-data-from-the-foundation-for-the-national-institute-of-healthosteoarthritis-initiative-biomarkers-consortium-to-evaluate-the-clinical-utility-of-prognostic-knee-osteoarth/. Accessed .« Back to 2016 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/statistical-simulation-using-data-from-the-foundation-for-the-national-institute-of-healthosteoarthritis-initiative-biomarkers-consortium-to-evaluate-the-clinical-utility-of-prognostic-knee-osteoarth/
