Session Information
Session Type: ACR Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Synovial biopsies are increasingly performed in both clinical setting and translational research. Synovial tissue analysis role in prediction of response to treatment is to be yet considered and regarding that most studies involve multicentric participation, an unmet need is the standardization of synovial biopsies procedures.
The aim of this collaborative work was to create a consensual set of items for handling and analysis of synovial biopsies in clinical practice and
translational research.
Methods: EULAR Synovitis Study Group (ESSG) and Synovial Tissue Special Interest Group (SIG) members were consulted through a Delphi survey. 3 sequential rounds occurred between June 2016 and June 2017. The items were identified and formulated based on a comprehensive literature review. Members were sent a written questionnaire containing items divided in 2 parts. The first part of the questionnaire referred to clinical practice containing 5 subsections: biopsy sampling, biopsy handling, histological analysis, staining and immunohistochemistry (IHC), biopsy analysis and pathologistÕs report. The second part referred to translational research and contained 6 subsections (same 5 plus RNA analysis).
Every participant was asked to score each item with a 5 points Likert (0: strongly disagree, 5: strongly agree), comments were allowed for each item. Items with a median score above 3.5 on 5 and a percentage of agreement above 70% were for the next round. Items with lower score were either suppressed of modified according to participantsÕ comments. Anonymized detailed results were circulating through participants between each round. Last round occurred orally at ESSG meeting in June 2017.
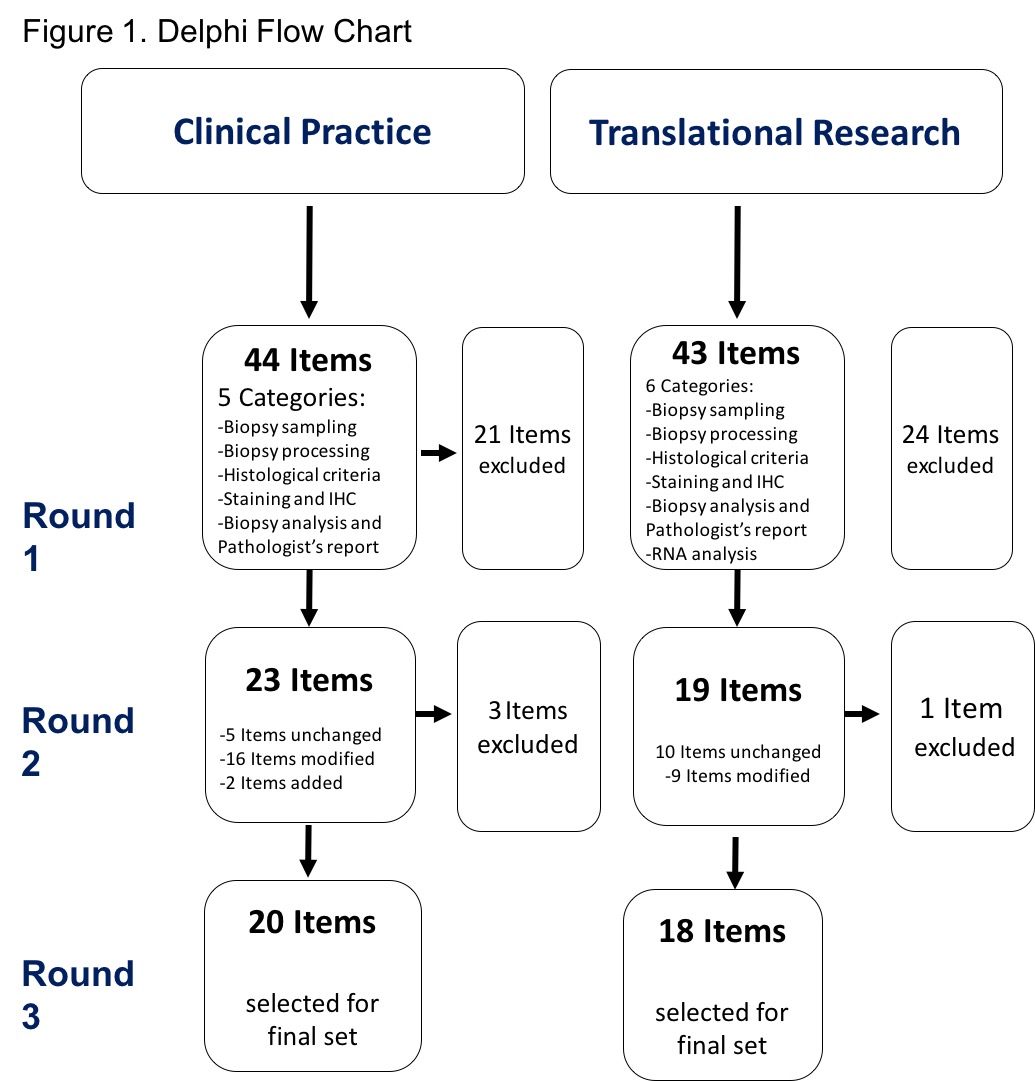
Results: 27 ESSG members from 19 centers were contacted by email. 20 participants from 17 centers answered (response rate of 74%). Response rates for next rounds were 100%. First questionnaire contained 44 items for Part 1 Clinical practice and 43 items for the second part about translational research. The flow chart is described in Figure 1. Third oral round allowed to obtain a final set of items unanimously (Figure 2).
Conclusion: We hereby propose a set of consensual points to consider on analysis of synovial biopsies in clinical practice and translational research to be further used in multicentric clinical trials and therefore ensure reliability.
To cite this abstract in AMA style:
Najm A, Le Goff MD PhD B, Orr C, Thurlings R, Cañete JD, Humby F, Alivernini S, Just SA, Romão VC, Krenn V, Müller-Ladner U, Addimanda O, Manzo A, Tas SW, Durez P, Meric de Bellefon L, Stoenoiu M, Strand V, Wechalekar MD, Fonseca JE, Lauwerys BR, Veale DJ. Standardization of Synovial Biopsies Procedures across Centers: A Consensus Initiative Using a Delphi Survey [abstract]. Arthritis Rheumatol. 2018; 70 (suppl 9). https://acrabstracts.org/abstract/standardization-of-synovial-biopsies-procedures-across-centers-a-consensus-initiative-using-a-delphi-survey/. Accessed .« Back to 2018 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/standardization-of-synovial-biopsies-procedures-across-centers-a-consensus-initiative-using-a-delphi-survey/