Session Information
Session Type: Abstract Session
Session Time: 5:00PM-5:50PM
Background/Purpose: Remission is the preferred treatment target in rheumatoid arthritis (RA), and many patients require biologic disease-modifying antirheumatic drugs (DMARDs) to reach this state. It is debated whether tapering of tumor necrosis factor inhibitor (TNFi) treatment to discontinuation should be considered in RA patients who sustain remission on treatment (1). The primary study objective was to assess the effect of tapering and withdrawal of TNFi on the risk of flares in RA patients in clinical remission.
Methods: In the non-inferiority ARCTIC REWIND trial, RA patients in remission for at least 12 months on stable TNFi therapy were randomly assigned to continued stable TNFi or tapering (half-dose TNFi for 4 months, thereafter withdrawal of TNFi), with visits every four months. csDMARD co-medication was kept stable in both arms. Patients had to be in DAS remission at inclusion with 0 swollen joints of 44 assessed. The primary endpoint was the proportion of patients with disease flare during the 12-month study period (defined as DAS >1.6, change in DAS >0.6 and 2 or more swollen joints, or the physician and patient agreed that a clinically significant flare had occurred). Full-dose TNFi was reinstated in case of flares in the tapering arm. The non-inferiority margin was 20%, with a predefined superiority test if non-inferiority was not shown. The inferiority null-hypothesis was tested in the per-protocol population by mixed effect logistic regression. Radiographs were scored by van der Heijde modified Sharp score (0 and 12 months, average of two readers, progression: ≥1 unit change). Clinicaltrials NCT01881308.
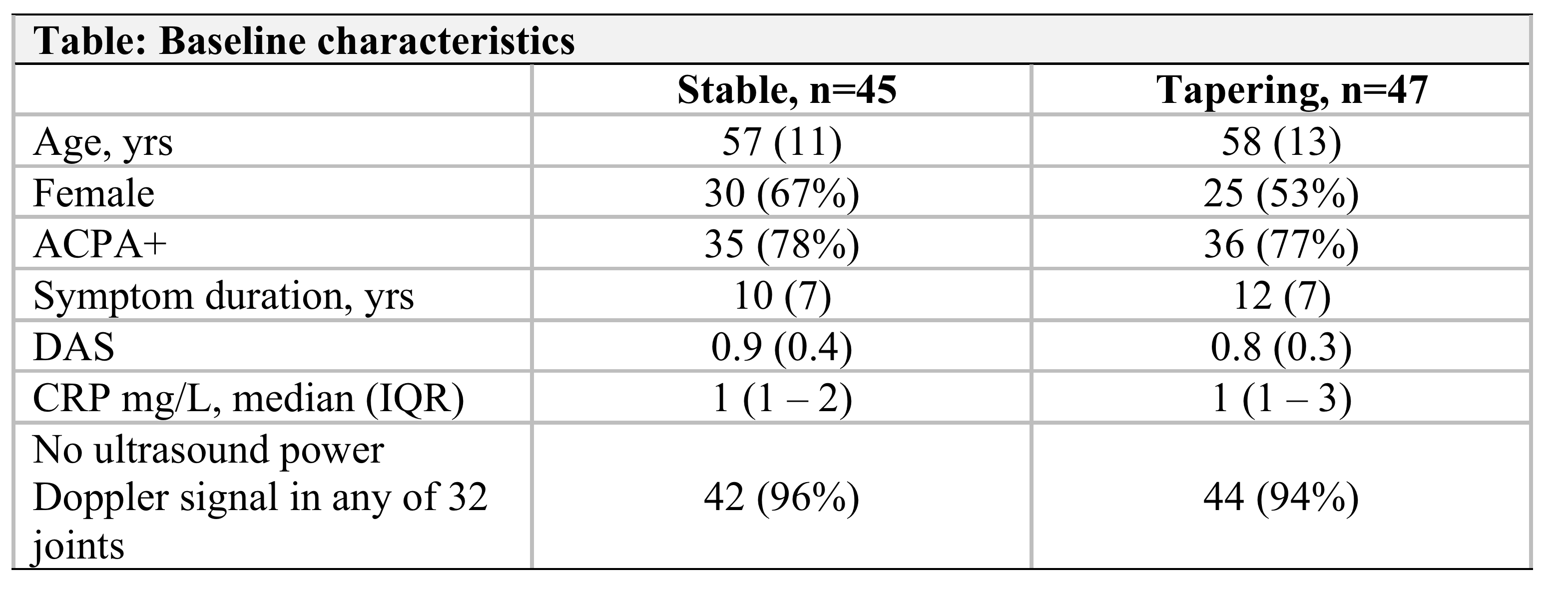
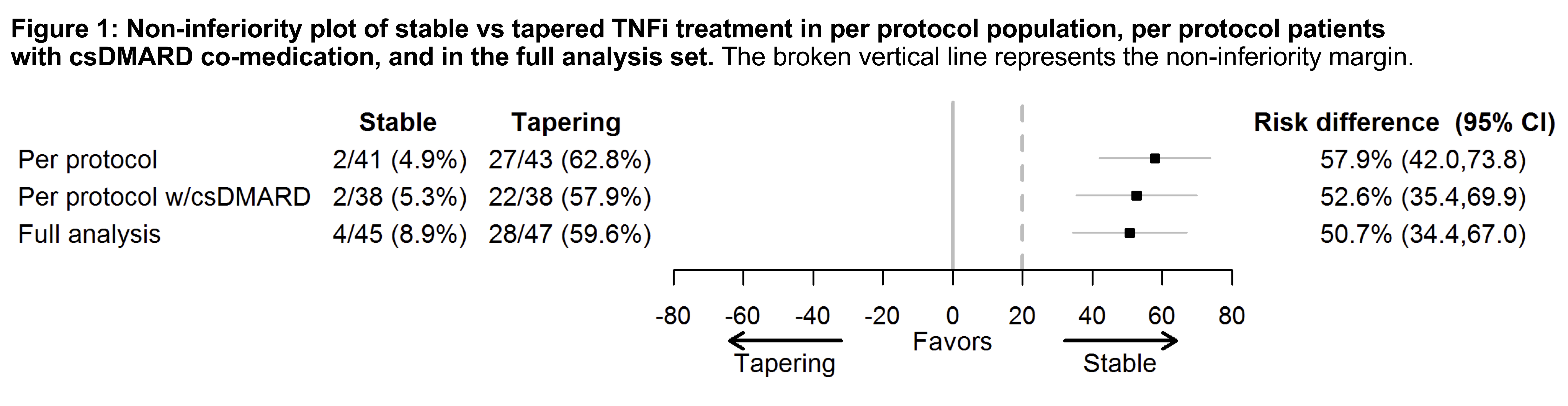
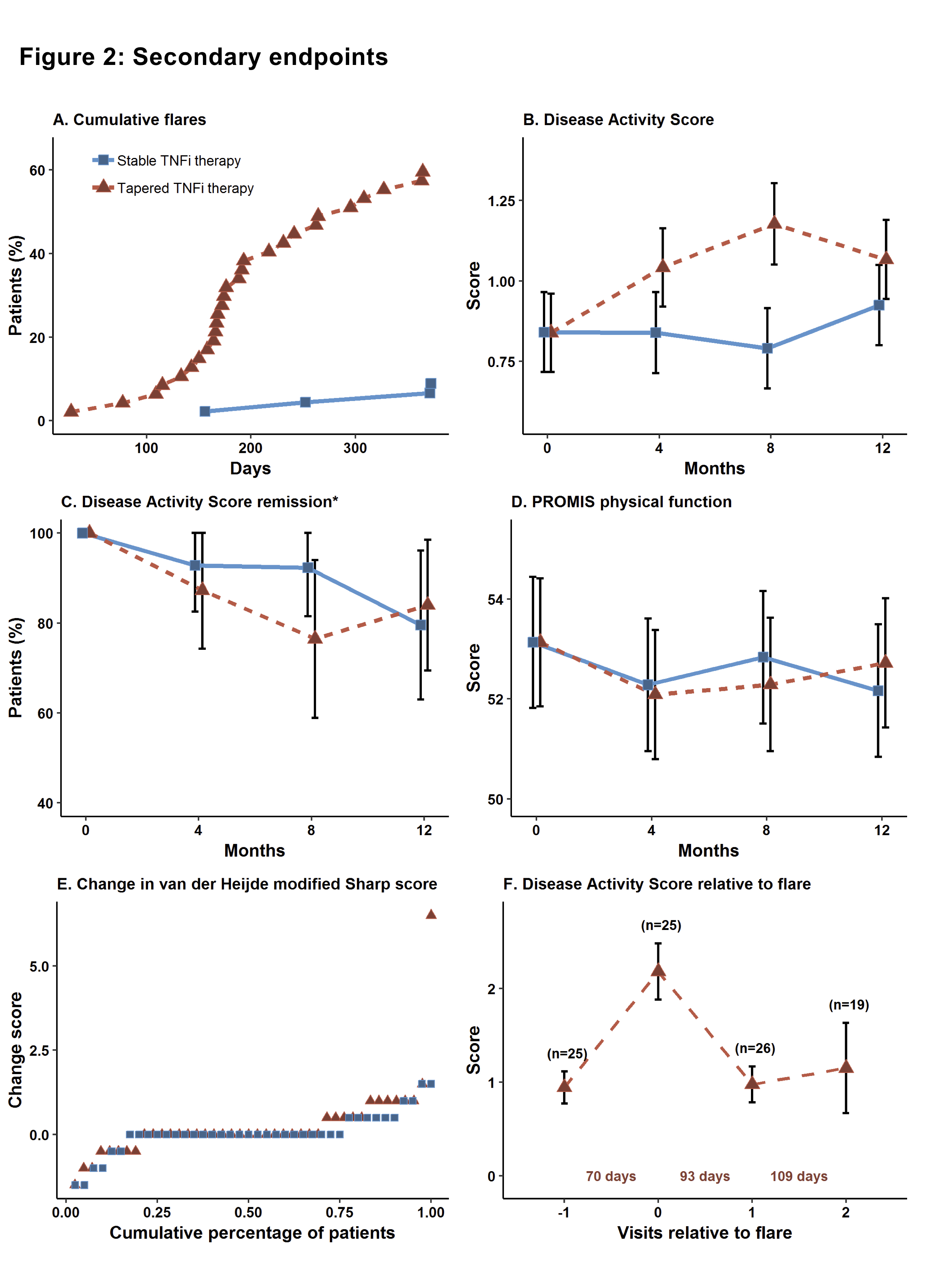
Results: We randomized 99 patients, 92 received the allocated treatment strategy, 84 were included in the per-protocol population. Baseline characteristics, clinical and ultrasound disease activity were balanced (Table). csDMARD co-medication was used by 93% in the stable and 88% in the tapering arm. In the primary analysis, 5% of patients in the stable TNFi arm experienced a flare during 12 months, compared to 63% in the tapering TNFi arm. The risk difference (95% CI) was 58% (42% to 74%, Fig 1), with stable treatment being deemed superior to tapering. 90% in the stable and 81% in the tapering arm did not show progression of radiographic joint damage, difference (95% CI) -9% (-24%, 6%). At 12 months, DAS scores, DAS remission and function were similar between groups (Fig 2). The numbers of adverse events (AE)/serious AE in the stable and tapering arm were 57/2 and 50/3, respectively, with 26 and 15 infections.
Conclusion: In a randomized clinical trial assessing patients in prolonged and deep RA remission, we observed a large increase in the flare rate in patients who tapered and discontinued TNFi. Patients responded well to reinstated treatment and remission rates in the two study arms were comparable at 12 months.
REFERENCES:
[1] Smolen et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. ARD 2020
To cite this abstract in AMA style:
Lillegraven S, Sundlisæter N, Aga A, Sexton J, Olsen I, Lexberg �, Madland T, Fremstad H, Høili C, Bakland G, Spada C, Haukeland H, Hansen I, Moholt E, Uhlig T, Solomon D, van der Heijde D, Kvien T, Haavardsholm E. Stable versus Tapered and Withdrawn Treatment with Tumor Necrosis Factor Inhibitor in Rheumatoid Arthritis Remission: A Randomized, Open-Label, Phase 4, Non-Inferiority Trial [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/stable-versus-tapered-and-withdrawn-treatment-with-tumor-necrosis-factor-inhibitor-in-rheumatoid-arthritis-remission-a-randomized-open-label-phase-4-non-inferiority-trial/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/stable-versus-tapered-and-withdrawn-treatment-with-tumor-necrosis-factor-inhibitor-in-rheumatoid-arthritis-remission-a-randomized-open-label-phase-4-non-inferiority-trial/