Session Information
Date: Monday, October 22, 2018
Title: 4M090 ACR Abstract: Systemic Sclerosis & Rel D/O–Clinical II: Clinical Trials II (1875–1880)
Session Type: ACR Concurrent Abstract Session
Session Time: 2:30PM-4:00PM
Background/Purpose: Interstitial lung disease (ILD) is the leading cause of death in systemic sclerosis (SSc). While some SSc-ILD patients are stable or improve with immunosuppressive therapy, others have progressive decline and currently available clinical parameters do not reliably predict outcomes. We investigated whether two specific pneumoproteins, Krebs von den Lungen-6 [KL-6] and CC chemokine ligand 2 [CCL18]) predict response to immunosuppression with cyclophosphamide (CYC) and mycophenolate (MMF) in SSc-ILD.
Methods: 142 patients in Scleroderma Lung Study (SLS) II were randomized to receive either mycophenolate (MMF) for 2 years or oral CYC for 1 year followed by 1 year of placebo. All available serum baseline and 12-month samples were investigated. CCL18 was assayed by commercially available ELISA while KL-6 was measured using antibody coated latex microbeads and an automated analyzer. The forced vital capacity (FVC) and the diffusing capacity for carbon monoxide (DLCO) were measured every 3 months. Quantitaive Lung fibrosis (QLF) and Quantitative ILD (QILD) scores for whole lung (WL) and zone of maximum involvement (ZM) were measured at baseline. To investigate the relationship between baseline CCL18 and KL-6 and progression of ILD, joint models were created with the outcomes of the course of the FVC and DLCO over 1 year.
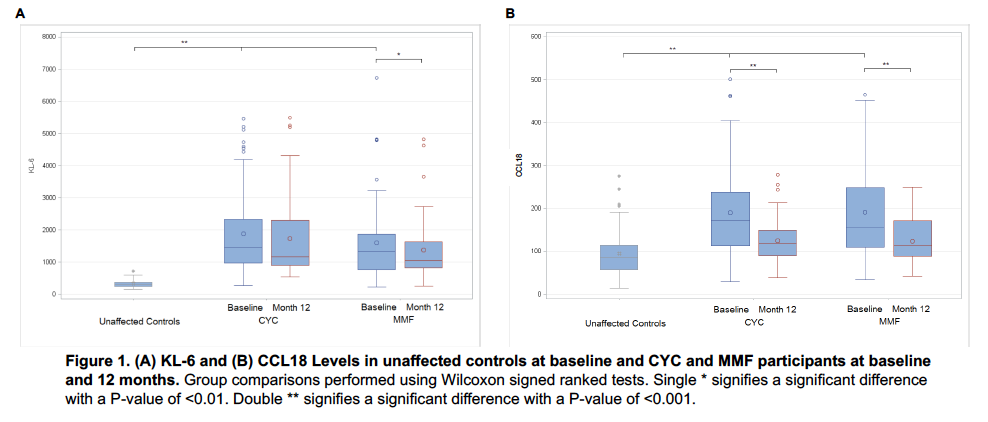
Results: Baseline serum KL-6 and CCL18 correlated with extent of ILD. KL-6 levels correlated with (r, P-value): DLCO (-0.3; 0.0002) QLF-WL (0.5; <0.0001) QLF-ZM (0.4; <0.0001), and QILD-WL(0.5; <0.0001), QILD-ZM (0.5; <0.0001); Baseline CCL18 levels were correlated with QILD-WL (r=0.2; p=0.04) and QILD-ZM (r=0.3; p=0.009), but not with the FVC or DLCO. CCL18 and KL6 levels declined significantly in the first year in both arms (Figure 1). After adjusting for baseline disease severity, higher baseline KL6 levels predicted progression of ILD as measured by the course of the FVC (CYC/MMF: Estimate -0.31/-0.74; P=0.024/<0.001) and DLCO (CYC/MMF: Estimate -1.30/-1.29; P<0.001/<0.001) over 1 year for both treatment arms. Similarly, higher baseline CCL18 levels predicted progression of ILD as measured by the course of the FVC (CYC/MMF: Estimate -1.24/-0.35; P<0.001;0.007) and DLCO (CYC/MMF: Estimate -1.87/-1.24; P=0.001/0.002) over 1 year for both treatment arms.
Conclusion: In the context of a rigorously conducted clinical trial for SSc-ILD, baseline KL-6 and CCL18 levels correlated with several surrogate measures of ILD severity. Patients with higher baseline KL-6 and CCL18 levels were more likely to experience disease progression despite treatment with CYC and MMF. KL-6 and CCL18 could be used to identify patients with progressive ILD that require closer monitoring in clinical practice. KL-6 and CCL18 may also be used to enrich SSc-ILD clinical trials with those at risk of accelerated ILD progression with conventional immunosuppressive therapy.
To cite this abstract in AMA style:
Volkmann ER, Tashkin DP, Kuwana M, Li N, Charles J, Hant FN, Bogatkevich GS, Akter T, Roth M, Kim HJG, Goldin J, Khanna D, Clements PJ, Furst DE, Elashoff R, Silver R, Assassi S. Specific Pneumoproteins Predict Progression of Interstitial Lung Disease in Systemic Sclerosis Patients Undergoing Treatment with Immunosuppression [abstract]. Arthritis Rheumatol. 2018; 70 (suppl 9). https://acrabstracts.org/abstract/specific-pneumoproteins-predict-progression-of-interstitial-lung-disease-in-systemic-sclerosis-patients-undergoing-treatment-with-immunosuppression/. Accessed .« Back to 2018 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/specific-pneumoproteins-predict-progression-of-interstitial-lung-disease-in-systemic-sclerosis-patients-undergoing-treatment-with-immunosuppression/