Session Information
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: Belimumab (BLM) is a monoclonal antibody that inhibits B-lymphocyte stimulating factor (BlyS), approved in 2011 as a treatment for systemic lupus erythematosus (SLE). We present the experience with BLM in this spanish cohort.
Methods: Descriptive, retrospective, multicenter study in patients diagnosed with SLE according to EULAR/ACR 2019, SLICC and/or ACR 1997 diagnostic criteria. Data regarding SLE patients treated with BLM were collected from medical records (2011-2023). Demographic features, analytical variables, SLEDAI, renal involvement, steroid dose, administration routes, efficacy, remission and safety were assessed.
Results: Five hundred and twenty one patients were included with a mean age at diagnosis of 35.1 (13.9) years. 85.8% were women. The mean age at BLM begins was 44.5 (13.3) years and the median time from SLE diagnosis to BLM begin was 6.5 (2.4-14.3) years. Regarding administration route 50.2% were on intravenous (IV), 34.6 % on subcutaneous (SC) and 15.2% change from IV to SC route. The mean time of follow-up was 24.0 (21.4) months.
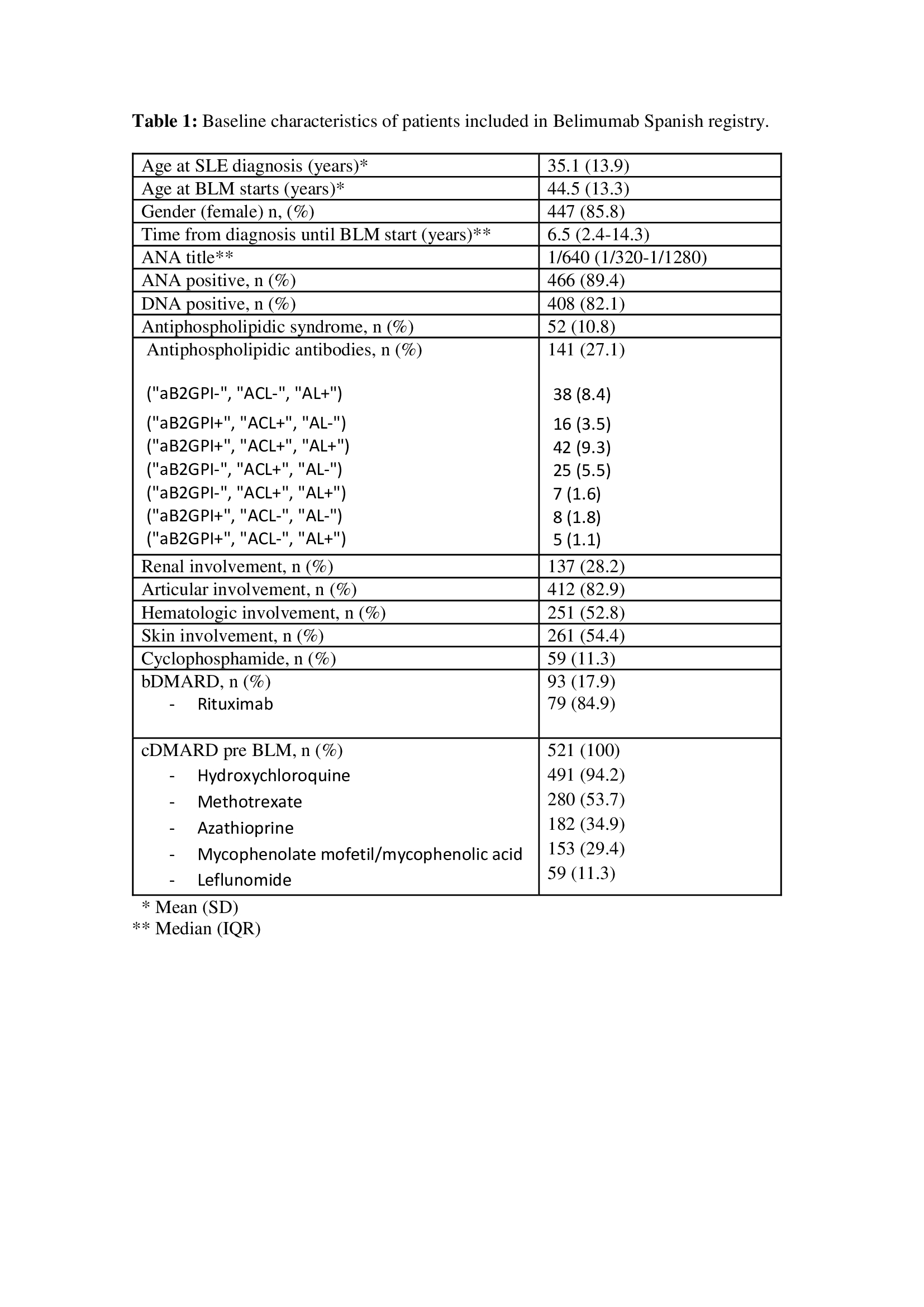
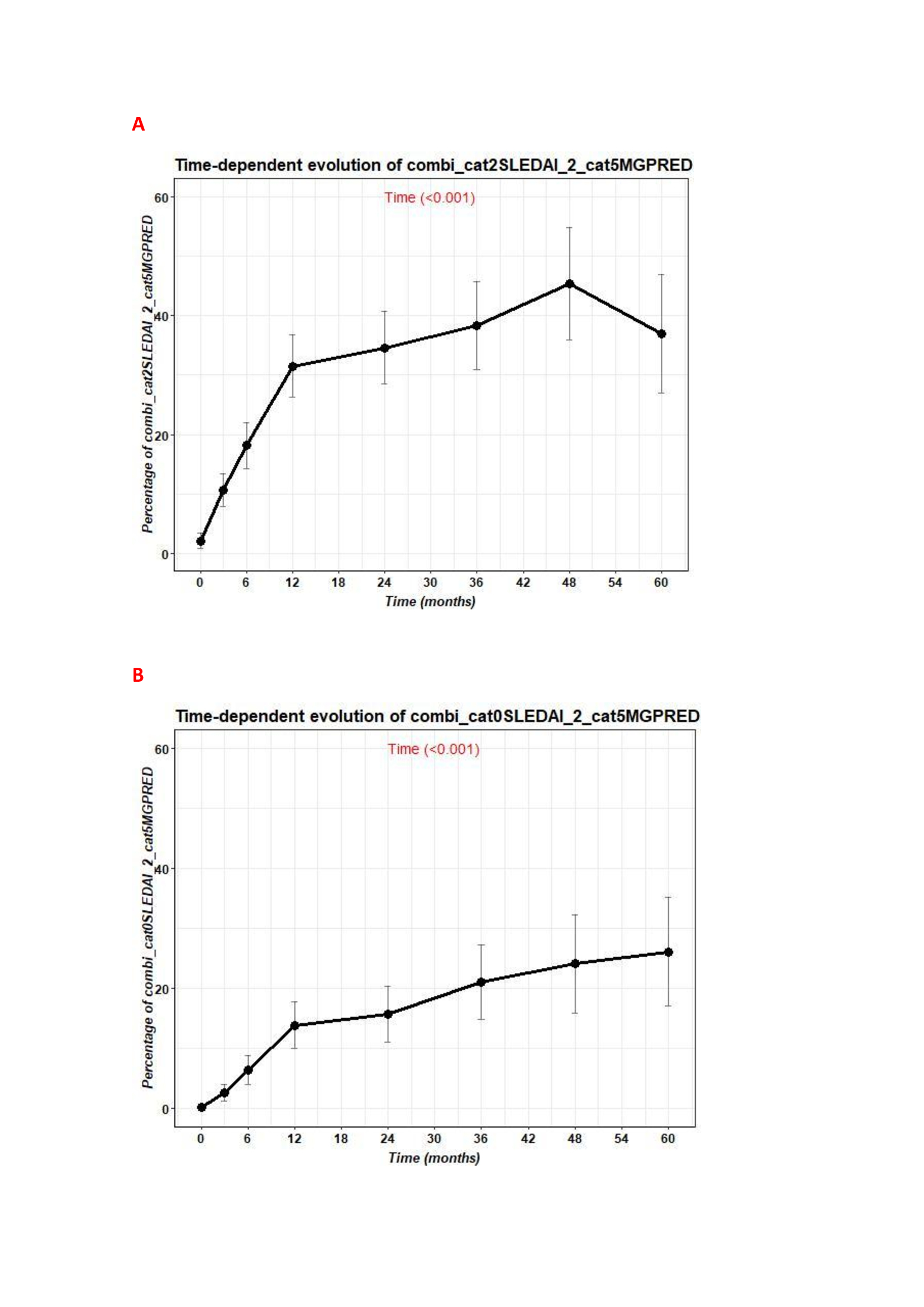
Baseline characteristics are summarized in Table 1 and patients in remission are shown in figure 1.
The median number of pre-BLM cDMARD use was 2.0 (2.0-3.0), being hydroxychloroquine (HCQ) the most used (94.2%). 93 patients received bDMARD before BLM begins, being Rituximab (RTX) the most used (79.6%), and 59 patients were treated with IV cyclophosphamide (CFM) with a median of 6 (4.5-8) IV bolus received. 75.6% and 21.5% of patients were on treatment with HCQ and methotrexate when BLM was started respectively.
The main reasons of starting BLM were musculoskeletal and mucocutaneous disorders (table1).
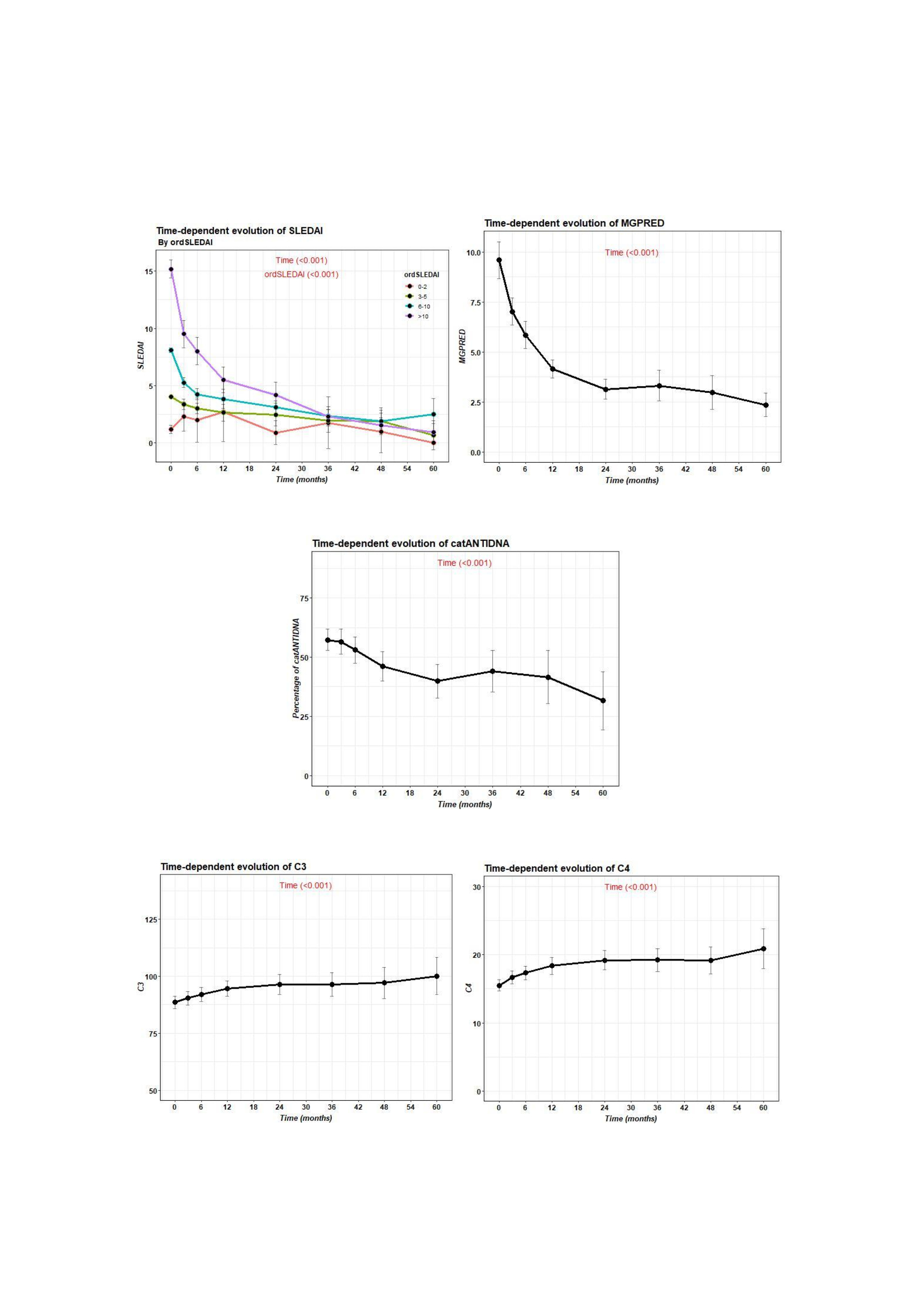
503 patients were on prednisone at the time of BLM begin with a median dose of 7.5 (5-10) mg. A statistically significant decrease in prednisone dose, SLEDAI and DNA was observed from baseline until the last follow up. In the other hand complement C3 and C4 raised (figure2).
One hundred and thirty seven patients (28.2%) had renal involvement. The median 24 hours proteinuria was 1.0 (0.5-2.4) grams and renal biopsy was done in 114 out of 137 patients, being class IV (42%), III (18.8%), II (12.5%) and V (10.7%) the most reported. 72.2% improve after BLM begins with last 24 hour proteinuria median of 0.2 (0.1-0.8) grams. Prior to BLM, most of them had been treated with MMF, RTX or CFM.
One hundred and and fitty one patients (29%) discontinued treatment mostly due to inefficacy (57.1%) and infections (12.2%). 133 patients developed infections, most of them mild, being urinary infection the most reported. 3 patients died, 8 had COVID and 3 presented neoplasms requiring discontinuation of the drug. The median time on treatment of patients that had to stop BLM was 12.0 (6-24) months.
Conclusion: Almost half of patients achieved remission after 4 years of BLM and a statistically significant improvement in prednisone dose reduction, SLEDAI, complement and antiDNA was demonstrated. Corticosteroids sparing effect with BLM was also observed.
BLM seems to be a good choice for patients with lupus nephritis since nearly three quarters of them normalized proteinuria values. No new safety alarms were reported.
This is the largest cohort of SLE patients treated with BLM reported in one country.
To cite this abstract in AMA style:
laiño M, Enguita M, Loricera J, Moriano Morales C, Lasa C, Calvo Río V, Narvaez J, Navarro Guerra P, Casafont-Sole I, Font Urgelles J, Gallego A, Carrión-Barberà I, Quiroga P, Castañeda S, Garcia-Aparicio A, Belzunegui Otano J, De Dios J, López M, Hernandez S, Heredia Martin S, Fariñas A, Navarro F, Fragio J, Escudero C, Ortega Castro R, del Olmo Perez L, Pinillos V, Labrador E, Ortega M, Castro Pérez P, Blanco-Madrigal J, Paulino M, Matías M, Calvo-Aranda E, Peralta C, García S, Camins-Fàbregas J, Garijo Bufort M, Fabregas D, Urruticoechea A, Medina Malone M, Cossio Jimenez P, Perez-Pampin E, Varas de Dios B, Vazquez Galeano C, Vegas Revenga N, Rusinovich O, Giner-Serret E, lamúa J, Aldasoro V. Spanish National Registry of Belimumab in Patients with Systemic Lupus Erythematosus [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/spanish-national-registry-of-belimumab-in-patients-with-systemic-lupus-erythematosus-2/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/spanish-national-registry-of-belimumab-in-patients-with-systemic-lupus-erythematosus-2/