Session Information
Date: Monday, November 13, 2023
Title: Plenary II
Session Type: Plenary Session
Session Time: 11:00AM-12:30PM
Background/Purpose: Sodium-glucose cotransporter-2 inhibitors (SGLT2) have benefits on kidney and cardiovascular (CV) outcomes that are largely independent of glycemic control. These benefits have been demonstrated in patients with type 2 diabetes, heart failure, and other causes of proteinuric kidney disease, but it is unknown whether SGLT2 have similar benefits in patients with SLE and lupus nephritis (LN).
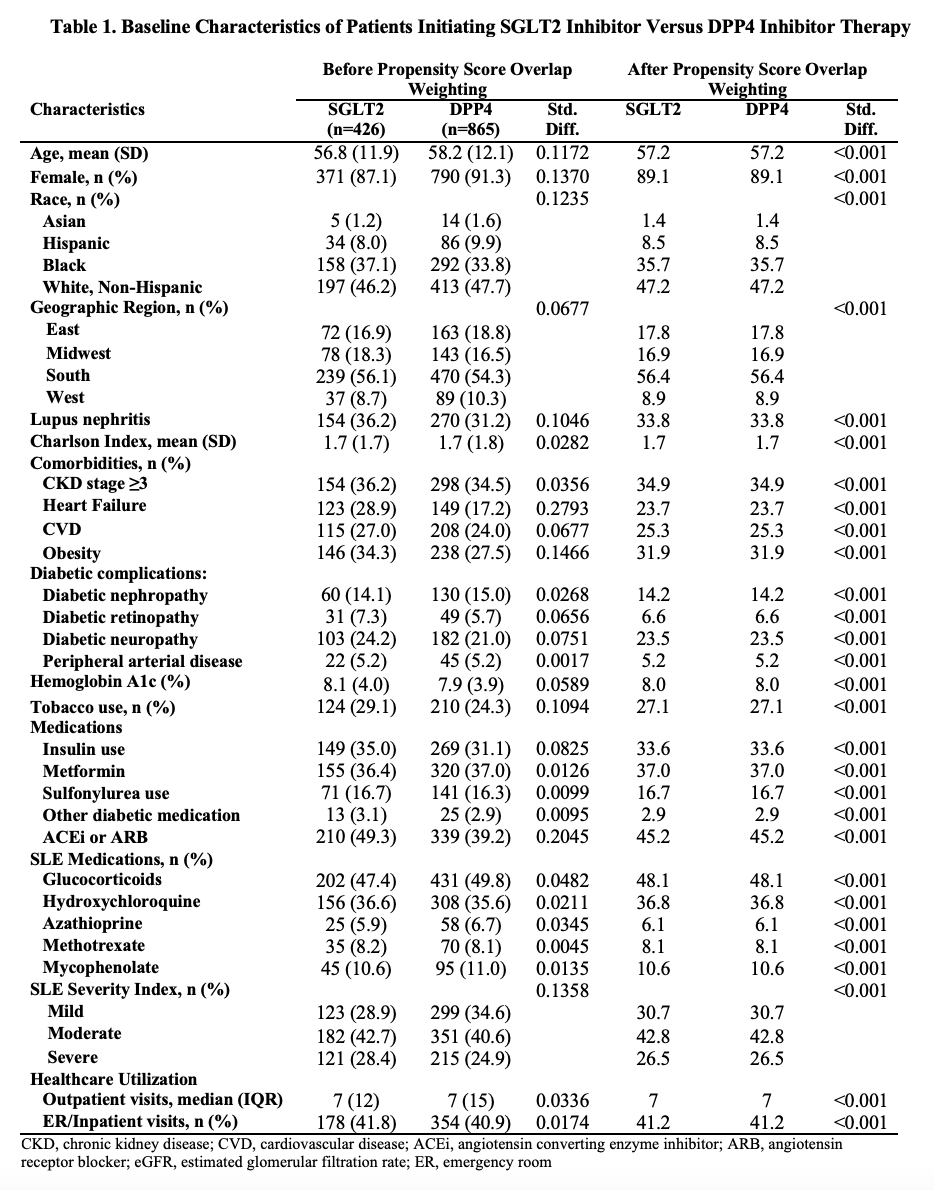
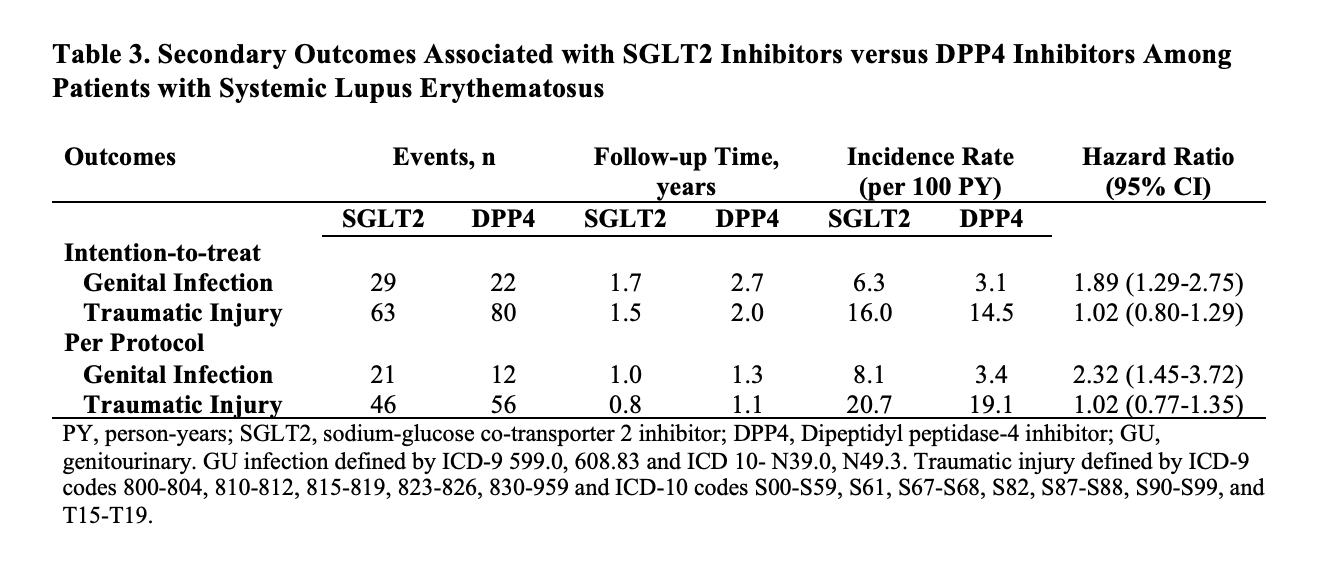
Methods: We designed and emulated a pragmatic target trial to determine the impact of SGLT2 versus a comparator oral hypoglycemic agent, dipeptidyl peptidase 4 inhibitors (DPP4), on kidney and CV outcomes among patients with SLE and LN. Using a large US multi-center electronic health record-based SLE cohort (N=96,511), we identified all patients with incident prescriptions for SGLT2 or DPP4 after diagnosis with SLE from 3/2013 to 8/2021. We used propensity score overlap weighting to balance baseline covariates including demographics, geographic region, LN, comorbidities (e.g., CKD, diabetes, heart failure), tobacco use, diabetic complications, HbA1c, medication use including other hypoglycemic agents, angiotensin converting enzyme inhibitors (ACEi)/angiotensin receptor blockers (ARBs), glucocorticoids, hydroxychloroquine, and immunosuppressants, SLE severity index, and healthcare utilization. We assessed the outcomes of renal progression, defined by a decrease in eGFR by ≥30% or new onset end-stage renal disease, and major adverse cardiac events (MACE), defined by CV death or hospitalization for ischemic stroke, myocardial infarction, heart failure, or venous thrombosis. We also assessed the safety outcome of genital infection and the control outcome of traumatic injury. We used Cox regression to assess the risk of each outcome, using an intention-to-treat analysis and a per-protocol analysis with censoring from deviation from treatment assignment.
Results: There were 426 and 865 incident users of SGLT2 and DPP4 with SLE. This included 154 and 270 patients with LN, respectively. After propensity score overlap weighting, all baseline covariates were balanced (Table 1). The mean age was 57 years; 89% were female. 36% were Black and 8% were Hispanic. 35% had CKD and 24% had heart failure. ACEi/ARBs were used by 45%. SGLT2 use was associated with a lower risk of MACE (HR 0.69 [95% CI 0.48-0.99]) and renal progression (HR 0.71 [95% CI 0.51-0.98]) than DPP4 use (Table 2). Among the subgroup with LN, the risk of MACE was also lower with SGLT2 use (HR 0.58 [95% CI 0.34-0.99]); there were numerical differences in renal progression but it did not reach statistical significance. SGLT2 us was associated with a higher risk of genital infections (Table 3). There was no difference in the risk of traumatic injury (HR 1.02 [0.80-1.29]) as expected.
Conclusion: In this cohort of patients with SLE who initiated SGLT2 or DPP4, we found a lower risk of MACE and renal progression associated with SLGT2i use, suggestive of cardiac and renal benefits as have been observed in other disease populations. Limitations include the small sample size and that patients in this study used SGLT2 for non-SLE indications. However, the findings indicate a potential role for SGLT2 for patients with SLE/LN and warrants further investigation in these populations.
To cite this abstract in AMA style:
Jorge A, Zhou B, McCormick N, Yokose C, Zhang Y, Choi H. Sodium-Glucose Co-transporter-2 Inhibitors and the Risk of Cardiac and Renal Outcomes in Systemic Lupus Erythematosus [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/sodium-glucose-co-transporter-2-inhibitors-and-the-risk-of-cardiac-and-renal-outcomes-in-systemic-lupus-erythematosus/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/sodium-glucose-co-transporter-2-inhibitors-and-the-risk-of-cardiac-and-renal-outcomes-in-systemic-lupus-erythematosus/