Session Information
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Sodium-glucose co-transporter-2 inhibitors (SGLT2-i) are anti-diabetic drugs that have a urate-lowering effect. SGLT2-i had a more favorable impact on gout risk than glucagon-like peptide-1 (GLP-1) receptor agonists. In a post-hoc analysis of a randomized controlled trial (RCT), canagliflozin, a SGLT2-i, decreased risk of gout that seemed to be more than may be expected by serum urate reduction, suggesting potential anti-inflammatory effects beyond urate reduction. In-vitro, canagliflozin, but not dapagliflozin or empagliflozin, activated AMPK and suppressed IL-1β stimulated secretion of IL-6 and monocyte chemoattractant protein-1 (MCP-1). However, dapagliflozin and empagliflozin activated AMPK in other studies. Whether there may be differences in gout risk between SGLT2-i’s is not known.
Methods: We performed a cohort study using data from The Health Improvement Network (THIN), a UK general practitioner (GP) electronic health records (EHR) database. We included subjects aged 18–89 with type 2 diabetes mellitus and incident use of a SGLT2-i (canagliflozin, dapagliflozin, empagliflozin) between 01/01/2013 and 12/ 31/2018 who had been enrolled in the GP practice for 6 months prior to study entry. We excluded individuals with gout diagnosis (Read code), use of gout medications (allopurinol, febuxostat, probenecid, sulfinpyrazone, or colchicine) or without body mass index assessment prior to SGLT2-i initiation. Follow-up started from the index date (date of first SGLT2-i prescription) and continued until incident gout diagnosis (diagnostic Read code or prescription of gout medications), switch to another SGLT2-i, death, or end of study. We compared the risk of developing gout across the 3 SGLT2-i’s using multivariable Cox proportional hazards regression, adjusting for potential confounders.
Results: Of 22,049 subjects with type 2 diabetes mellitus, there were 3,287 incident users of canagliflozin, 12,504 incident users of dapagliflozin and 6,258 incident users of empagliflozin. Baseline characteristics are shown in Table 1. After adjusting for potential confounders, canagliflozin had 1.57 (95% confidence interval (CI) of 1.18 to 2.09) times the risk of developing gout, while empagliflozin had 0.81 (95% CI 0.59–1.11) times the risk compared with dapagliflozin, although the latter the effect estimate was not statistically significant (Table 2).
Conclusion: While SGLT2-i agents as a class and canagliflozin in particular have been shown to reduce the risk of gout in prior studies, in this cohort study from a UK EHR database, the beneficial effects appeared to be strongest for dapagliflozin and empagliflozin, with no significant differences noted between the two of them. Although canagliflozin reduced risk of gout compared with placebo in a RCT, in these data, it had a less favorable effect than dapagliflozin. The clinical relevance of the in vitro data on the potential anti-inflammatory effects of the various agents in this drug class is not clear.
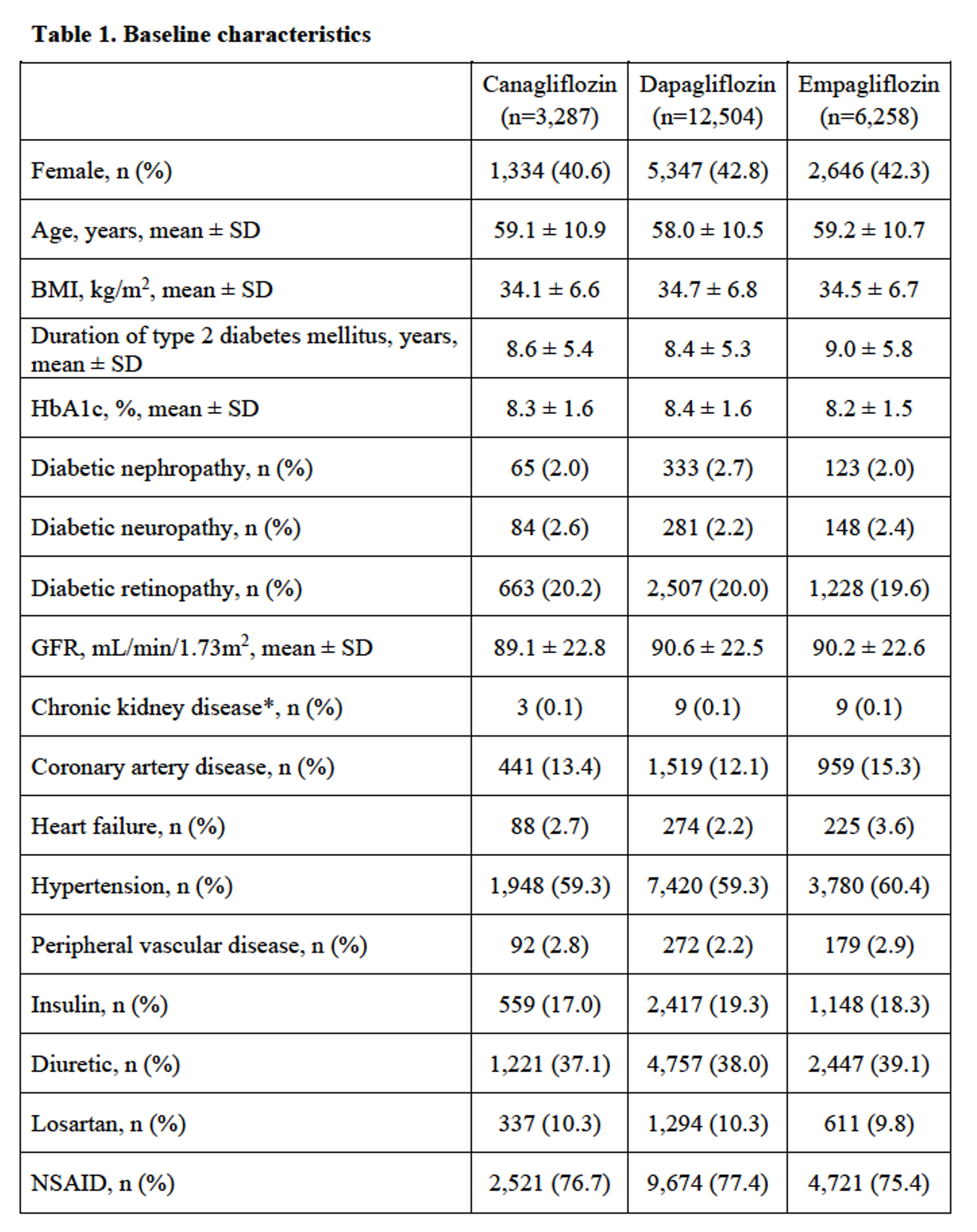 *Chronic kidney disease (CKD) defined as CKD stage 4, CKD stage 5, or renal replacement therapy (dialysis or transplant). BMI: body mass index; GFR: glomerular filtration rate; HbA1c: glycated hemoglobin; NSAID: non-steroidal anti-inflammatory drug; SD: standard deviation.
*Chronic kidney disease (CKD) defined as CKD stage 4, CKD stage 5, or renal replacement therapy (dialysis or transplant). BMI: body mass index; GFR: glomerular filtration rate; HbA1c: glycated hemoglobin; NSAID: non-steroidal anti-inflammatory drug; SD: standard deviation.
 * Potential confounders (assessed at baseline) included in the adjusted hazards ratio model: 1) general (age, gender, body mass index); 2) duration of type 2 diabetes mellitus; 3) complications of type 2 diabetes mellitus (diabetic nephropathy, diabetic neuropathy, diabetic retinopathy); 4) comorbidities (chronic kidney disease, coronary artery disease, heart failure, hypertension, peripheral vascular disease); 5) medication use (diuretics, insulin, losartan, non-steroidal anti-inflammatory drug). CI: confidence interval; SGLT2-i: sodium-glucose co-transporter 2 inhibitors.
* Potential confounders (assessed at baseline) included in the adjusted hazards ratio model: 1) general (age, gender, body mass index); 2) duration of type 2 diabetes mellitus; 3) complications of type 2 diabetes mellitus (diabetic nephropathy, diabetic neuropathy, diabetic retinopathy); 4) comorbidities (chronic kidney disease, coronary artery disease, heart failure, hypertension, peripheral vascular disease); 5) medication use (diuretics, insulin, losartan, non-steroidal anti-inflammatory drug). CI: confidence interval; SGLT2-i: sodium-glucose co-transporter 2 inhibitors.
To cite this abstract in AMA style:
Vargas-Santos A, Peloquin C, Kim S, Neogi T. Sodium-Glucose Co-Transporter-2 Inhibitors and the Risk for Gout – a Comparison Among Canagliflozin, Dapagliflozin and Empagliflozin [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/sodium-glucose-co-transporter-2-inhibitors-and-the-risk-for-gout-a-comparison-among-canagliflozin-dapagliflozin-and-empagliflozin/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/sodium-glucose-co-transporter-2-inhibitors-and-the-risk-for-gout-a-comparison-among-canagliflozin-dapagliflozin-and-empagliflozin/
