Session Information
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: A key treatment goal for patients (pts) with SLE is to reduce use of CS, due to their association with organ damage.1 BEL is an approved treatment for active SLE and LN, in addition to standard therapy (ST).1 BEL-treated pts were able to reduce CS dose and exhibited delayed organ damage progression during long-term extension studies (LTEs).2–4 We evaluated use of SLE medications and change in organ damage over time in pts with SLE using data from three multicenter LTE studies.
Methods: This post hoc analysis pooled data from the LBSL02 LTE (Phase 2; GSK Study 112626),2 BLISS-76 LTE (US pts only; Phase 3; GSK Study 112233),3 and BLISS-52 + BLISS-76 LTE (excluding US pts from BLISS-76; Phase 3; GSK Study 112234)4 studies. To enroll in LTEs, pts must have completed treatment through Week 72 of LBSL02 and BLISS-76, or Week 48 of BLISS-52; improvement in physician global assessment at Week 72 or 68 versus at first BEL dose was required in the LBSL02 LTE. Pts received BEL 10 mg/kg intravenously every 28 days plus ST at the start of each LTE, regardless of prior drug allocation. Maximum daily equivalent prednisone dose was summarized any time post–first BEL dose (in prior trial period or LTE) and in each year. SLICC/ACR Damage Index (SDI) score was assessed among eligible pts in the BLISS-76, and BLISS-52 + BLISS-76 LTE studies; the high disease activity (HDA) population (anti-dsDNA positive and low C3/C4); and the 5-year completer population (pts with ≥5 years of SDI data post–first BEL dose).
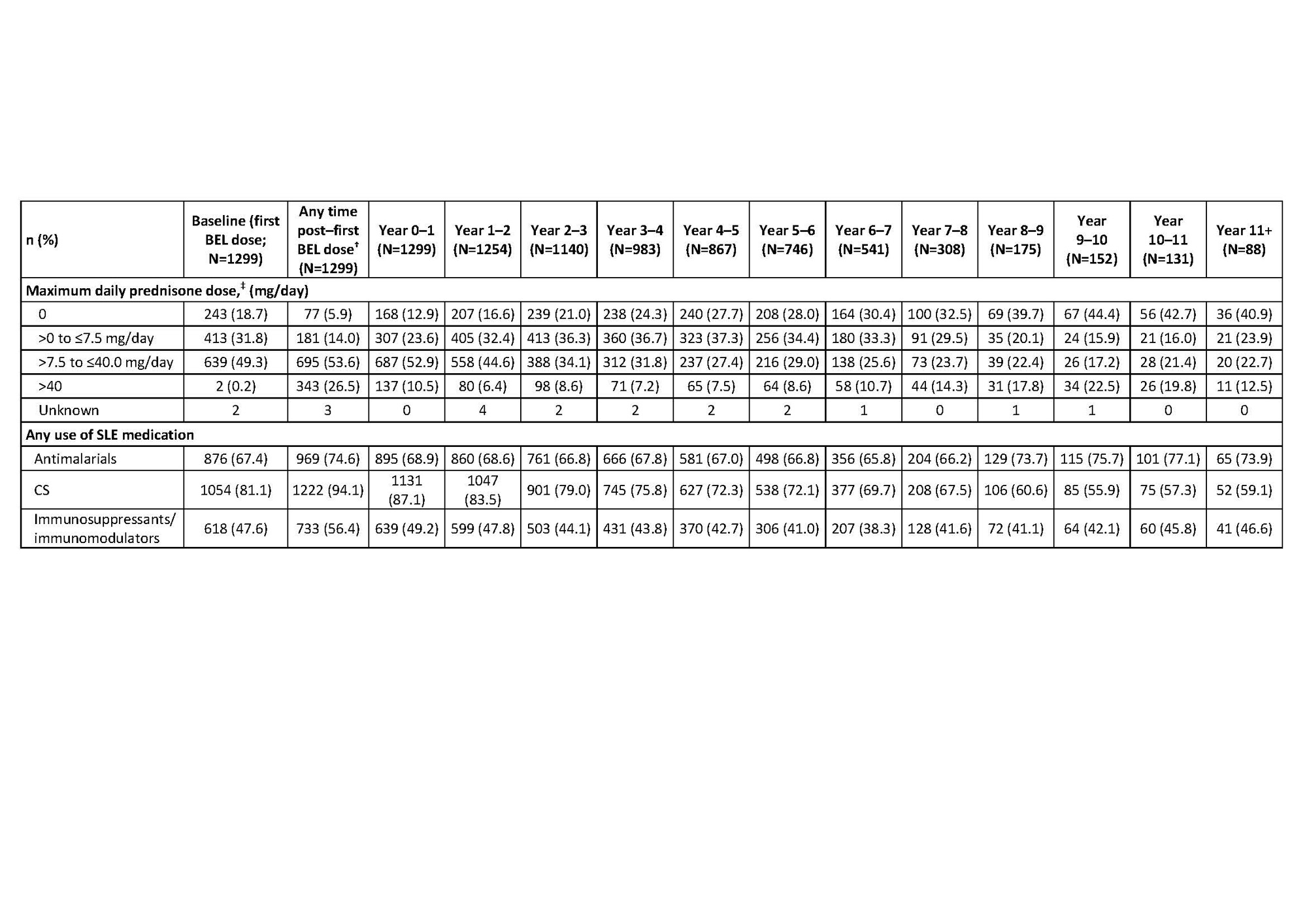
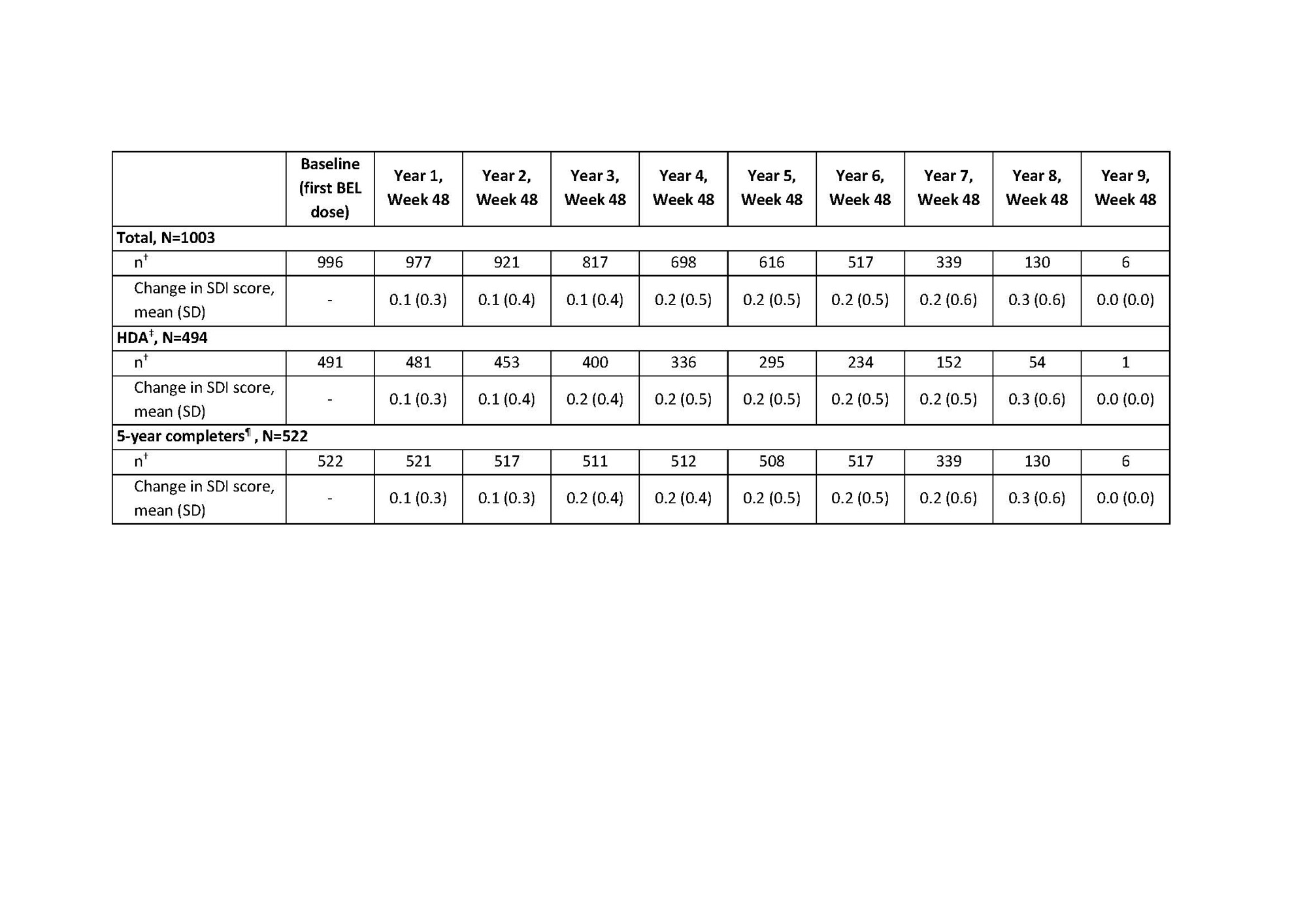
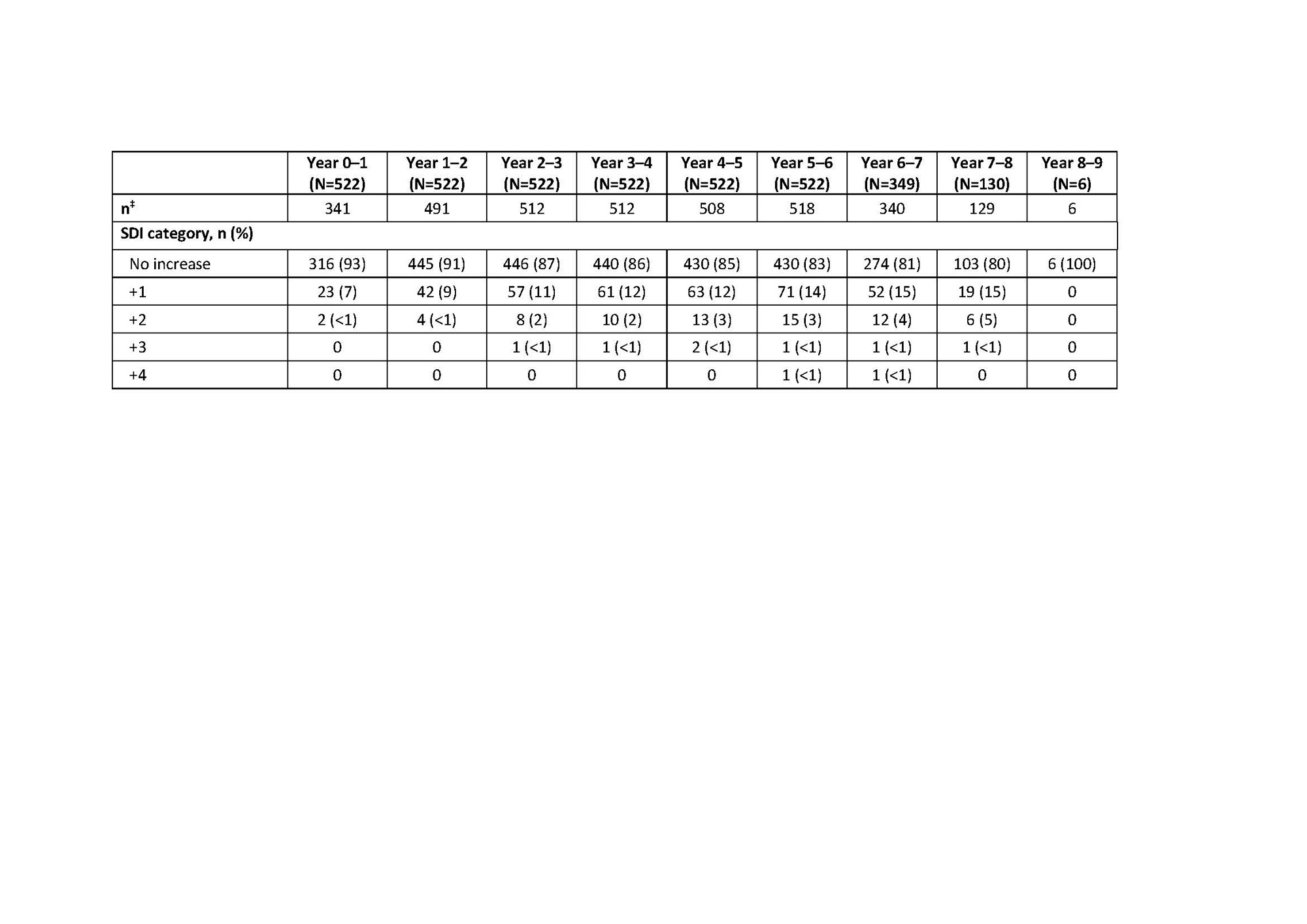
Results: Overall, 1304 pts enrolled in the LTE studies, 1299 (99.6%) received ≥1 dose of study drug (pooled safety population) and 604 (46.5%) completed their respective studies. Cumulative BEL-treated patient-years was 7040.1. At baseline (first BEL dose in prior trial period or LTE), 1054 (81.1%) pts received CS. Over time, the proportion of pts discontinuing CS (receiving an equivalent dose of 0 mg/day) increased; the proportion receiving >0 to ≤7.5 mg/day increased up to Year 4–5 (Table 1). Any use of CS decreased over time, while use of antimalarials and immunosuppressive/immunomodulatory agents remained stable (Table 1). At baseline, 580 (58.2%) and 416 (41.8%) pts had an SDI score of zero and ≥1, respectively. Mean (standard deviation [SD]) change over time in SDI score was similar and remained low for the total, HDA, and 5-year completer populations (Table 2). Among the 5-year completer population, ≤20% had SDI worsening (change ≥0) compared with baseline in any study year (Table 3).
Conclusion: Over time, more BEL-treated pts were able to reduce their daily equivalent prednisone dose and exhibited low change in SDI. Limitations include the open-label nature of the studies, lack of control arm, and increased number of withdrawals at later years. These data are consistent with previous observations of the steroid sparing–effect of BEL, and delayed organ damage progression among a large population of BEL-treated pts.2–5 References
1 Fanouriakis A et al. Ann Rheum Dis 2019;0:1–10
2 Wallace DJ et el. Arthritis Rheumatol 2019;7:1125–34
3 Furie RA et al. Arthritis Rheumatol 2018;6:868–77
4 van Vollenhoven RF et al. Rheumatology(Oxford) 2020;2:281–91
5 Urowitz MB et al. Lupus Sci Med 2020;7:e000412
Funding: GSK
*Data are summarized at any time post–first BEL dose and in each year; †post–first BEL dose data include follow-up visits after the final dose of BEL; data from Year 0 up to last visit in the treatment period are shown by years of study participation; pts could be counted in more than one year interval; ‡CS were converted to a daily prednisone equivalent dose and the maximum dose was summarized in each interval; maximum daily prednisone dose applies to any time post–first BEL dose and for each year interval; baseline daily prednisone dose was calculated as the average daily dose for the 7 days pre–first BEL dose.
*Observed data are presented; the SDI score is cumulative, once an item is scored, it continues to be scored at subsequent visits; for years in which a patient withdraws from the study, the exit visit assessment is used in place of the Week 48 assessment for the year, but this value is not carried forward through later years; †number of pts with available data at baseline and Week 48 yearly visit; ‡HDA is defined as anti-dsDNA positive and low C3/C4; ¶the 5-year completer population is defined as pts with ≥5 years of SDI assessment data post–first BEL dose (in prior trial period or LTE).
* Observed data are presented; the SDI score is cumulative, once an item is scored, it continues to be scored at subsequent visits; for years in which a patient withdraws from the study, the exit visit assessment is used in place of the Week 48 assessment for the year, and this value is not carried forward through later years; †the 5-year completer population is defined as pts with ≥5 years of SDI assessment data post–first BEL dose (in prior trial period or LTE); ‡number of pts with available data at baseline and year interval.
To cite this abstract in AMA style:
Touma Z, Arriens C, Henning C, Carroll A, Curtis P, Levy R, Stohl W. SLE Medication Usage and Organ Damage Among Adult SLE Patients with SLE Treated with Belimumab (BEL): Pooled Data from Three Open-Label Extension Studies over 11+ Years [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/sle-medication-usage-and-organ-damage-among-adult-sle-patients-with-sle-treated-with-belimumab-bel-pooled-data-from-three-open-label-extension-studies-over-11-years/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/sle-medication-usage-and-organ-damage-among-adult-sle-patients-with-sle-treated-with-belimumab-bel-pooled-data-from-three-open-label-extension-studies-over-11-years/