Session Information
Session Type: Poster Session B
Session Time: 9:00AM-10:30AM
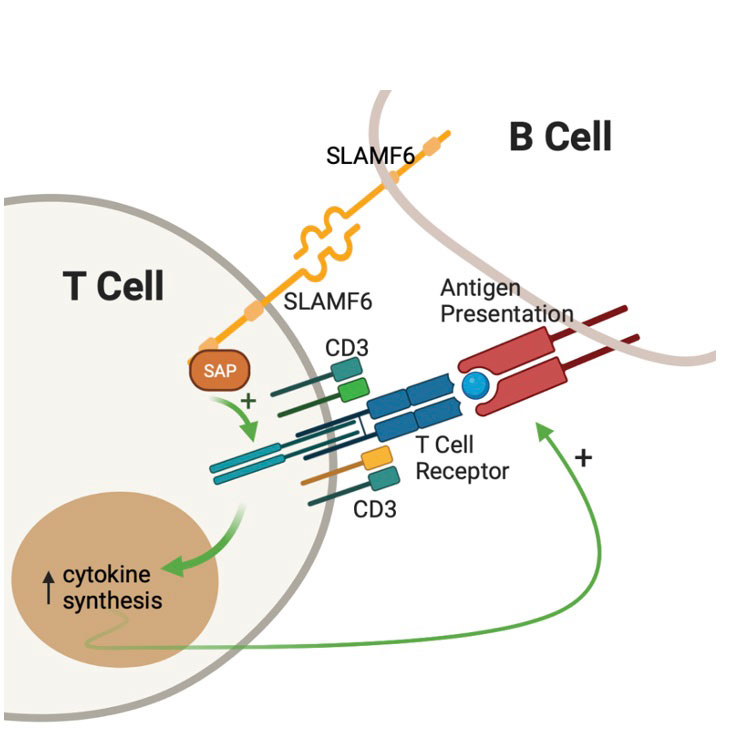
Background/Purpose: SLE is a multiorgan disease in which immune cells lack self-tolerance resulting in autoimmunity. SLE T cells infiltrate organs, provide help to autoreactive B cells and mediate inflammatory response. Signaling Lymphocyte Activation Molecule 6 (SLAMF6) is a T cell co-receptor whose gene is a risk locus for SLE both in murine models and humans. Following SLAMF6 receptor ligation, the intracellular signal is transmitted and amplified by the SLAM Associated Protein (SAP) –Fig1. SLAMF6-SAP signaling is critical for healthy T-B cell interactions, germinal center formations, and B cell maturation. This study was initiated to evaluate the role of SLAMF6-SAP in SLE.
Methods: Peripheral blood mononuclear cells (PBMCs) were isolated from whole blood using density gradient separation. Immunophenotyping using flow cytometry was performed after cells were labeled with anti-CD3 (FITC), anti-CD4 (AF700), anti-CD8 (BV605), anti-CXCR5 (PE-Cy7), anti-PD1 (BV421) and either anti-SLAMF6 or anti-SAP (APC). PBMCs were activated with anti-CD3 ± anti-SLAMF6 for 24 hours, after which IL-2 levels were analyzed by ELISA. RNA sequencing data from SLE patients was accessed from Gene Expression Omnibus (GEO) and analyzed for SAP gene (SH2D1A) expression.
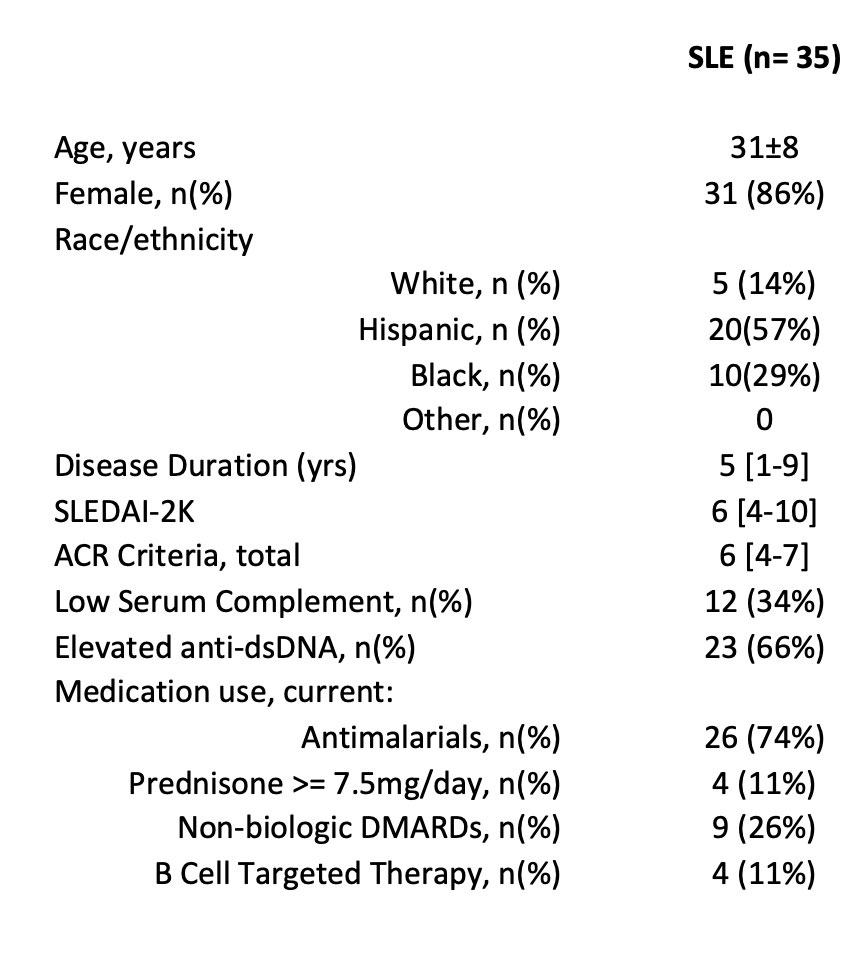
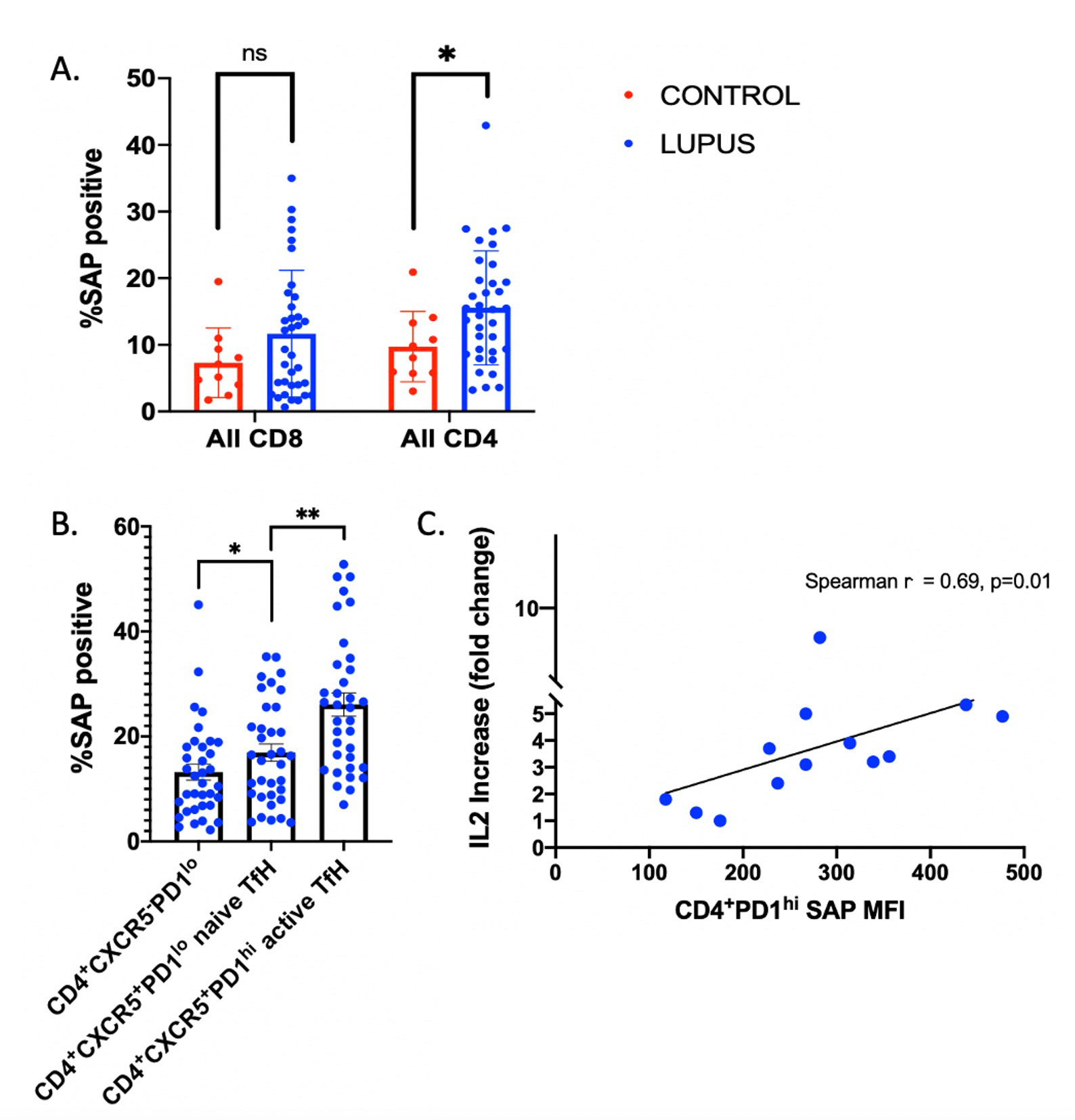
Results: We enrolled 35 patients with SLE (1997 ACR criteria), ages 31±8, 86% female. Median SLE disease duration was 5 [1-9] years. Median SLEDAI was 6 [4-10]; 34% had low complements and 66% had elevated anti-dsDNA. 74% were taking antimalarials, 11% prednisone 7.5mg/day, 26% non-biologic DMARDs, and 11% B-cell targeted therapy. Detailed data is presented in Table 1. SLAMF6 was not differentially expressed between SLE and healthy control CD4 T cells (93.7% and 93.2%, respectively, p=0.9). Conversely, SAP expression was increased in SLE CD4, but not CD8, T cells as compared to healthy controls (15.6% vs 9.8% p< 0.05 and 11.7% vs 7.3% p=0.2, respectively) -Fig 2A. SAP levels were increased in CD4+CXCR5+ T-follicular helper cells (TfH) as compared to CD4+CXCR5– cells (p=0.03), with still higher SAP levels seen in the activated TfH subgroup, defined by PD-1 expression (26.1% of CD4+CXCR5+PD1hi vs. 13.2% of CD4+CXCR5+PD1lo cells, p< 0.001) -Fig 2B. Stimulation of PBMCs with anti-CD3 + anti-SLAMF6 vs. anti-CD3 alone resulted in fold change IL-2 increase that correlated with CD4+PD1hi SAP expression (p=0.01) -Fig 2C. RNA sequencing data identified increased SAP expression in SLE as compared to control groups.
Conclusion: SLE T cells express greater SAP levels that result in enhanced SLAMF6 signaling. This is especially pronounced in the TfH cells, highlighting the role of TfH in SLE pathogenesis and identifying SLAMF6 as a potentially novel therapeutic target in the future.
To cite this abstract in AMA style:
Gartshteyn Y, Khalili L, Mor A, Askanase A. SLAMF6-SAP Signaling Unit Is Increased in SLE T Follicular Helper Cells [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/slamf6-sap-signaling-unit-is-increased-in-sle-t-follicular-helper-cells/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/slamf6-sap-signaling-unit-is-increased-in-sle-t-follicular-helper-cells/