Session Information
Date: Sunday, November 7, 2021
Title: Abstracts: Sjögren's Syndrome – Basic & Clinical Science (0984–0987)
Session Type: Abstract Session
Session Time: 3:45PM-4:00PM
Background/Purpose: Sjӧgren’s syndrome (SS) patients have lower quality of life driven by symptoms of pain, depression and fatigue. These symptoms often do not respond to traditional immunosuppressive therapy and may not be effectively addressed by physicians, driving a discordance between patient and provider expectations. Classification of SS patients into symptom-based categories has the potential to increase patient-provider harmony and impact therapeutic strategies. Our objective was to define relevant differences between clusters with respect to symptoms, treatment, disease activity, and laboratory profile.
Methods: We used the Sjögren’s International Collaborative Clinical Alliance (SICCA) registry to analyze 1,541 adults fulfilling the 2016 ACR/EULAR criteria for SS. Unsupervised hierarchical clustering was applied to identify the optimal phenotypically similar clusters by: 1) dryness (a composite score derived from Vitali C et al. 2002 consensus criteria ranging 0-100), 2) fatigue (5-point Likert scale from “not at all” to “nearly every day”), and 3) pain (5-point Likert scale from “not at all” to “extremely”). We reported demographic features, symptoms, medication use, organ involvement and laboratory values within each cluster. We used ANOVA or Chi-square to identify cluster differences, controlling for recruitment site, age, sex, race, ethnicity and education.
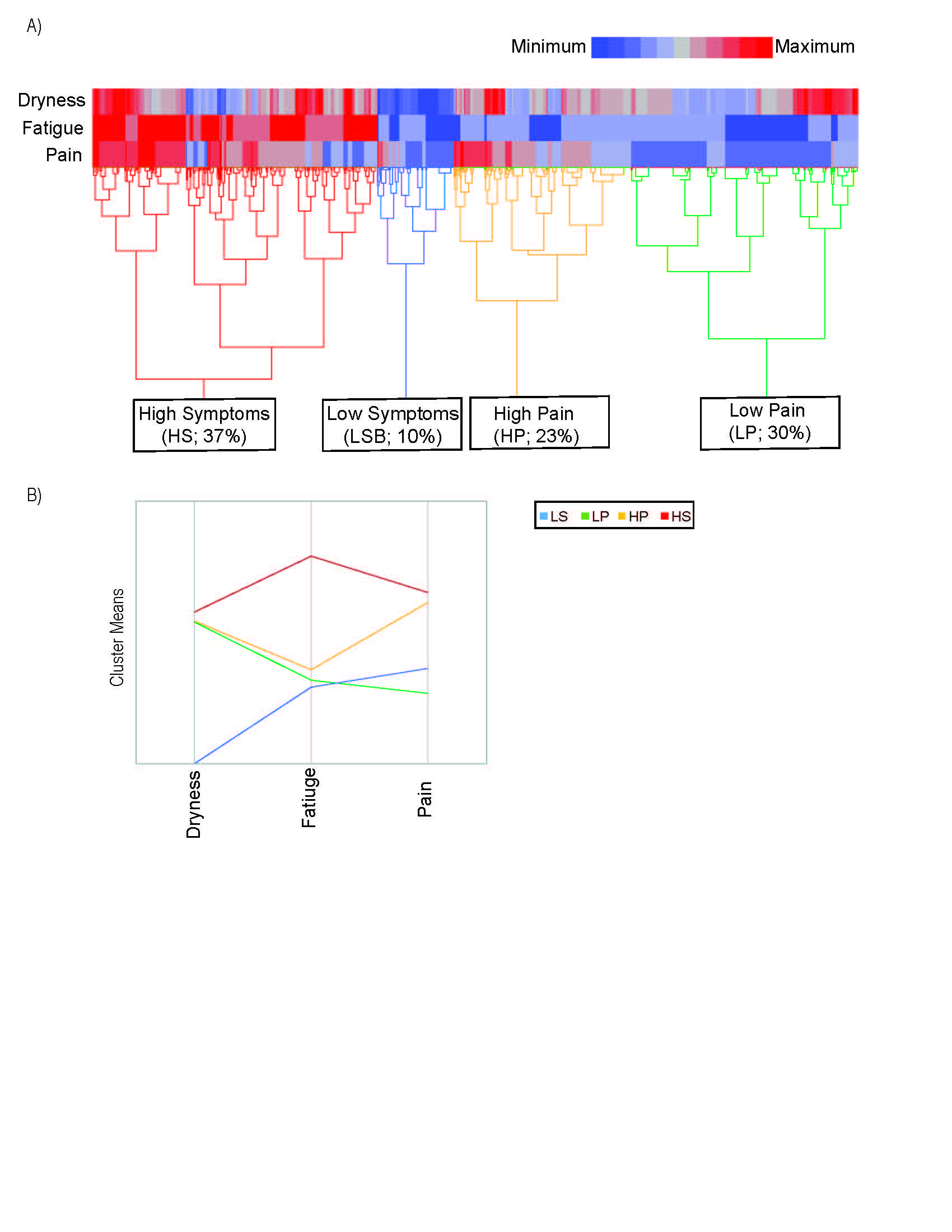
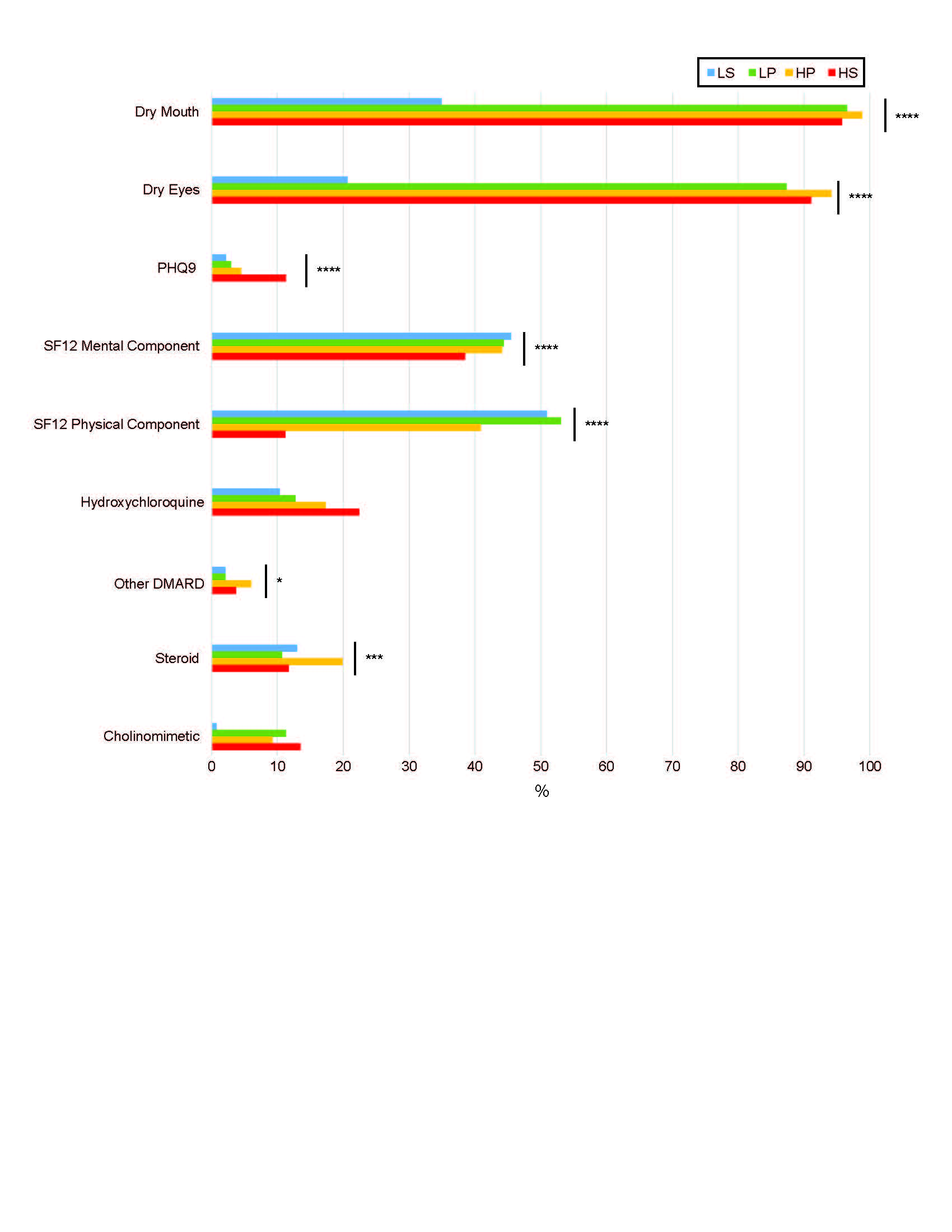
Results: The analysis yielded 4 clusters among 1,454 participants with complete data on the 3 key symptoms (Figure 1). Clusters were characterized by low symptom (LS; 10% prevalence) and high symptom frequency/severity (HS; 37%) in all categories; high pain (HP; 23%) and low pain (LP; 30%). Depression, measured by mean PHQ9, was worse in the HS group (11.3) compared to the LS group (2.2) (p< 0.0001). Health related quality of life, measured by the short form (SF) 12 (6 questions related to physical health, 5 question to mental health, and 1 question to both), was best in the LS group (mental) and LP group (physical) (Figure 2). The HP group was treated with corticosteroids and DMARDs most (20% & 6%). Extraglandular manifestations occurred most often in the LP group, though not reaching significance due to low frequencies of these manifestations (Figure 3A). Interestingly, synovitis occurred most frequently in the HP group (11%). Both the LS and LP groups had higher IgG than the HS and HP groups (Figure 3 B&C). Rheumatoid factor and focus scores were highest in the LP group (Figure 3 D&E).
Conclusion: Here we identify four clusters of SS patients based on dryness, pain and fatigue severity and show that symptom burden does not correlate well with traditional disease markers of severity. Additionally, treatments differed by cluster. The HP group received DMARDs and steroids most often; however, the LP group had the most prominent organ involvement and laboratory abnormalities, yet received immune modulating medication less frequently than the HS and HP groups. These findings highlight discordance between disease activity and treatments; symptoms drive therapies as opposed to disease activity. We propose future research defining these subsets to optimize treatment approaches in order to harmonize patient-provider expectations.
LP=low pain with some dryness and low fatigue; HP=high pain with some dryness and low fatigue; HS=high symptoms of dryness, fatigue
and pain; IgG=immunoglobulin G; RF=Rheumatoid Factor; *=adjusted p <0.05, **=adjusted p <0.01, ***=adjusted p <0.001, ***=adjusted p <0.0001. Models adjusted for age, sex, race, ethnicity, education, and recruitment site.
To cite this abstract in AMA style:
McCoy S, Woodham M, Saldanha I, Akpek E, BUNYA V, Baer A. Sjӧgren’s Symptom Burden Drives Immunomodulatory Therapies but Correlates Poorly with Disease Severity Markers [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/sj%d3%a7grens-symptom-burden-drives-immunomodulatory-therapies-but-correlates-poorly-with-disease-severity-markers/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/sj%d3%a7grens-symptom-burden-drives-immunomodulatory-therapies-but-correlates-poorly-with-disease-severity-markers/