Session Information
Session Type: Plenary Session II
Session Time: 11:00AM-12:30PM
Background/Purpose: Hand osteoarthritis (OA) is a prevalent joint disease with high disease-burden in need for effective therapeutic options. Studies have shown that synovial inflammation is often present in hand OA and a main determinant of pain and radiographic disease progression. Our aim was to investigate the efficacy and safety of short-term low-dose prednisolone in patients with painful hand OA.
Methods: This randomised, double-blind, placebo-controlled trial enrolled patients with painful hand OA, fulfilling American College of Rheumatology criteria, and signs of synovial inflammation. Patients with ≥4 interphalangeal joints (IPJ) with osteoarthritic nodes, ≥1 IPJ with soft swelling or erythema and ≥1 IPJ with positive power Doppler signal (PDS) or synovitis grade ≥2 on ultrasound, were eligible. Key exclusion criteria were chronic inflammatory rheumatic diseases, psoriasis, using immune modulating drugs within 90 days before baseline, and predominant thumb base pain. Eligible patients with visual analogue scale (VAS) finger pain ≥30 mm, flaring ≥20 mm upon non-steroidal anti-inflammatory drug washout, were randomised to receive prednisolone 10 mg daily for 6 weeks or placebo, followed by a two-week tapering scheme and 6 weeks without study medication. Outcomes were assessed at 2, 4, 6, 8 and 14 weeks. Primary endpoint was VAS finger pain at week 6 in intention-to-treat analysis. Secondary clinical endpoints included fulfilment of OMERACT-OARSI responder criteria, Australian/Canadian Hand OA Index (AUSCAN) pain/function, Functional Index for Hand OA (FIHOA), VAS patient global assessment, Short-Form 36 and grip strength. Imaging endpoints included ultrasound synovitis and PDS.
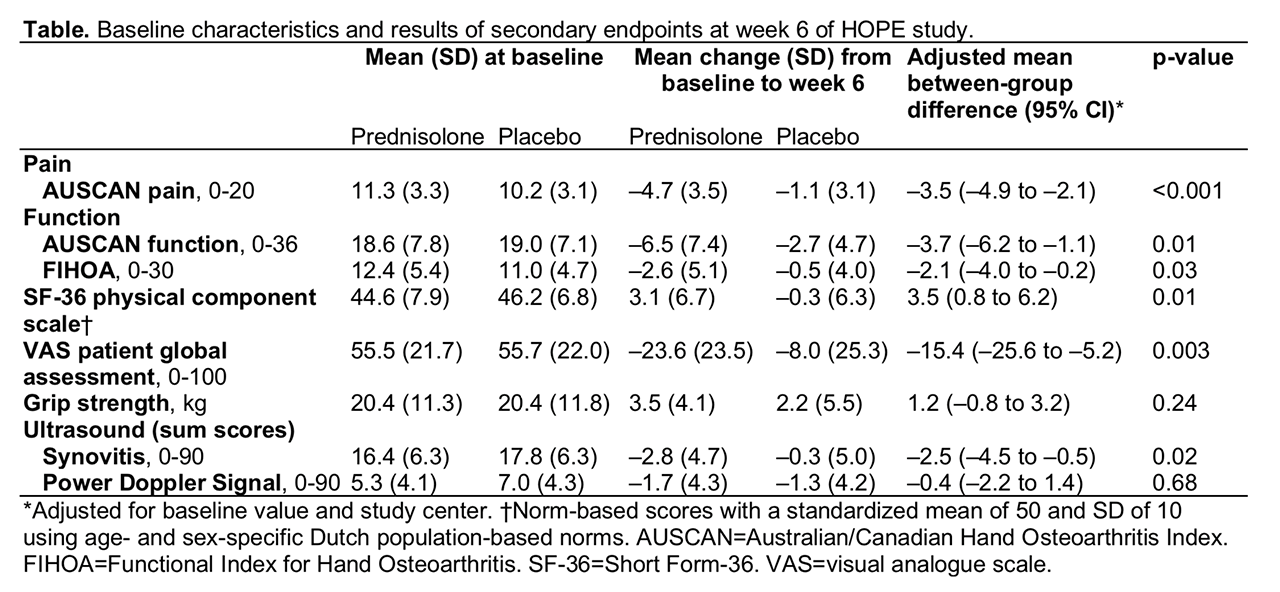
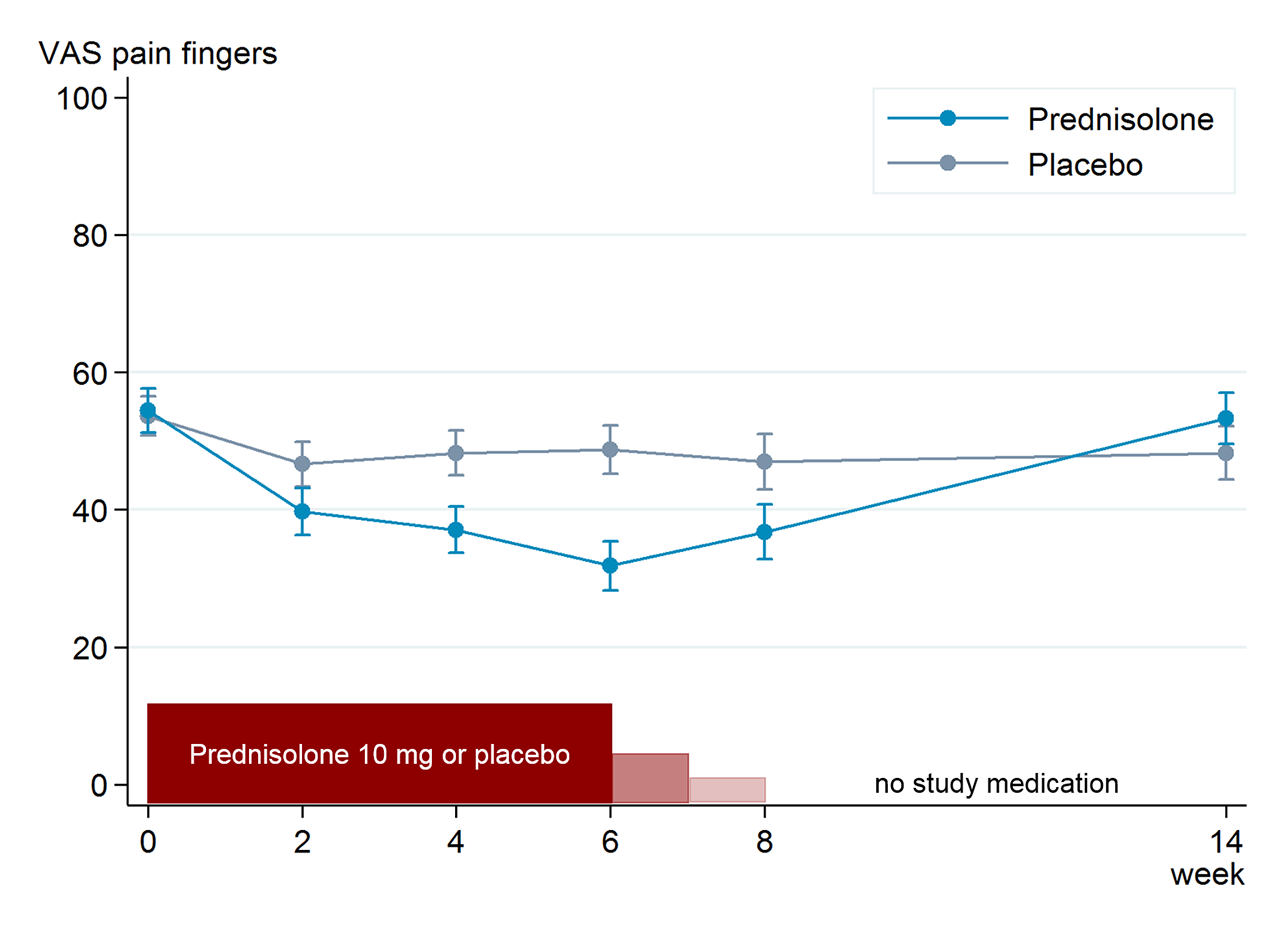
Results: Of 92 patients (mean (SD) age 63.9 (8.8), 79% women) randomised to prednisolone (n=46) or placebo (n=46), 42 patients in each group completed the study. Baseline characteristics were well-balanced between the groups. The mean (SD) change from baseline to week 6 in VAS finger pain was -21.5 (21.7) in the prednisolone and -5.2 (24.3) in the placebo group, with a mean between-group difference of -16.5 (95% confidence interval (CI) -26.1 to -6.9; figure). At week 6, 33 (72%) patients in the prednisolone versus 15 (33%) in the placebo group fulfilled OARSI responder criteria (odds ratio 5.3, 95% CI 2.0 to 13.6, p=0.001). In analogy with the primary endpoint, prednisolone was superior to placebo in most other secondary clinical endpoints (table). Ultrasound synovitis significantly improved at week 6 in the prednisolone compared to the placebo group, while no difference was observed in PDS (table). After tapering, between-group differences disappeared. Adverse events were mostly mild and comparable between groups.
Conclusion: Six-week treatment with low-dose oral prednisolone led to a substantial improvement of symptoms in patients with painful hand OA and signs of inflammation. This trial provides evidence that local inflammation is a suitable target for drug-treatment in hand OA.
To cite this abstract in AMA style:
Kroon F, Kortekaas M, Boonen A, Böhringer S, Reijnierse M, Rosendaal F, Riyazi N, Starmans M, Turkstra F, van Zeben J, Allaart C, Kloppenburg M. Six-week Treatment with Low-dose Prednisolone in Patients with Painful Hand Osteoarthritis (HOPE): Results from a Randomised Double-blind Placebo-controlled Trial [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/six-week-treatment-with-low-dose-prednisolone-in-patients-with-painful-hand-osteoarthritis-hope-results-from-a-randomised-double-blind-placebo-controlled-trial/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/six-week-treatment-with-low-dose-prednisolone-in-patients-with-painful-hand-osteoarthritis-hope-results-from-a-randomised-double-blind-placebo-controlled-trial/