Session Information
Date: Tuesday, October 28, 2025
Title: (1830–1854) Systemic Lupus Erythematosus – Etiology and Pathogenesis Poster
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Lupus nephritis (LN) is a severe complication of SLE that can progress to chronic kidney disease, renal fibrosis, and eventual renal failure. Fibroblasts activated by pro-inflammatory cytokines and damage signals adopt a heterogeneous pro-inflammatory or profibrotic phenotype, which contribute to pathological fibrosis; however, the fibroblast landscape in autoimmune kidney disease remains poorly understood. Here, using renal biopsies collected through the AMP-SLE, we define and localize fibroblast heterogeneity in LN through single-cell and spatial transcriptomics.
Methods: Single-cell RNA-sequencing was used to profile dissociated kidney biopsies collected from 156 LN patients and 30 controls. High-resolution clustering was performed to identify both tissue and immune cell subsets. 16,187 PDGFRB+ stromal cells were used for further subclustering. Covarying Neighborhood Analysis (CNA) was performed to identify statistically significant Pearson’s correlations between the frequencies of cell subsets and the ISN chronicity and activity indices. Spatial transcriptomics was performed using the 10x Genomics Xenium platform, with a gene panel customized to allow the identification of high-resolution myeloid populations.
Results: We identified fibroblasts, myofibroblasts, CNN1+ vascular smooth muscle cells/pericytes (VSMC/peri), mesangial cells and renal endothelial cells, with relative expansion of fibroblasts, myofibroblasts, and mesangial cells occurring during LN together with relative contraction of CNN1- VSMC/peri (Fig.1A, B). Fine clustering of myofibroblasts revealed two transcriptionally distinct myofibroblast-like populations with increased collagen scores compared to other fibroblast-like subsets: proinflammatory myofibroblasts (Myofib1) and profibrotic myofibroblasts (Myofib2). CNA revealed that both clusters of myofibroblasts and the VCSM/Peri1 subset were positively correlated with chronicity (Fig. 1C), but not with the activity index. Gene expression and pathway enrichment analyses highlighted MyoFib2 as a matrix-producing, contractile population, while MyoFib1 was enriched for immune signaling and complement pathways. PDGFRA+, ACTA2+ C7+ ITGA3- Myofib1 myofibroblasts were located preferentially in the interstitium adjacent to CSF1R+ resident macrophages, whereas PDGFRA, ACTA2+, ITGA3+ VCAN+ Myofib2 myofibroblasts were located in sclerotic areas of the glomeruli and fibrous crescents adjacent to GPNMB+ FABP5+ damage-associated infiltrating macrophages (Fig. 2A-C). Vascular mural cells also showed heterogeneity: VCSM/Peri1 was enriched for immune-related genes and expressed a detachment profile, whereas VCSM/Peri2–6 retained a contractile phenotype.
Conclusion: Our findings reveal the diversity of PDGFRB+ populations in LN kidneys and the presence of both proinflammatory and profibrotic myofibroblasts in various renal compartments associated with LN chronicity. Previously described LN associated resident and infiltrating macrophages co-localize with proinflammatory and profibrotic fibroblasts respectively creating unique renal niches that promote tissue damage.
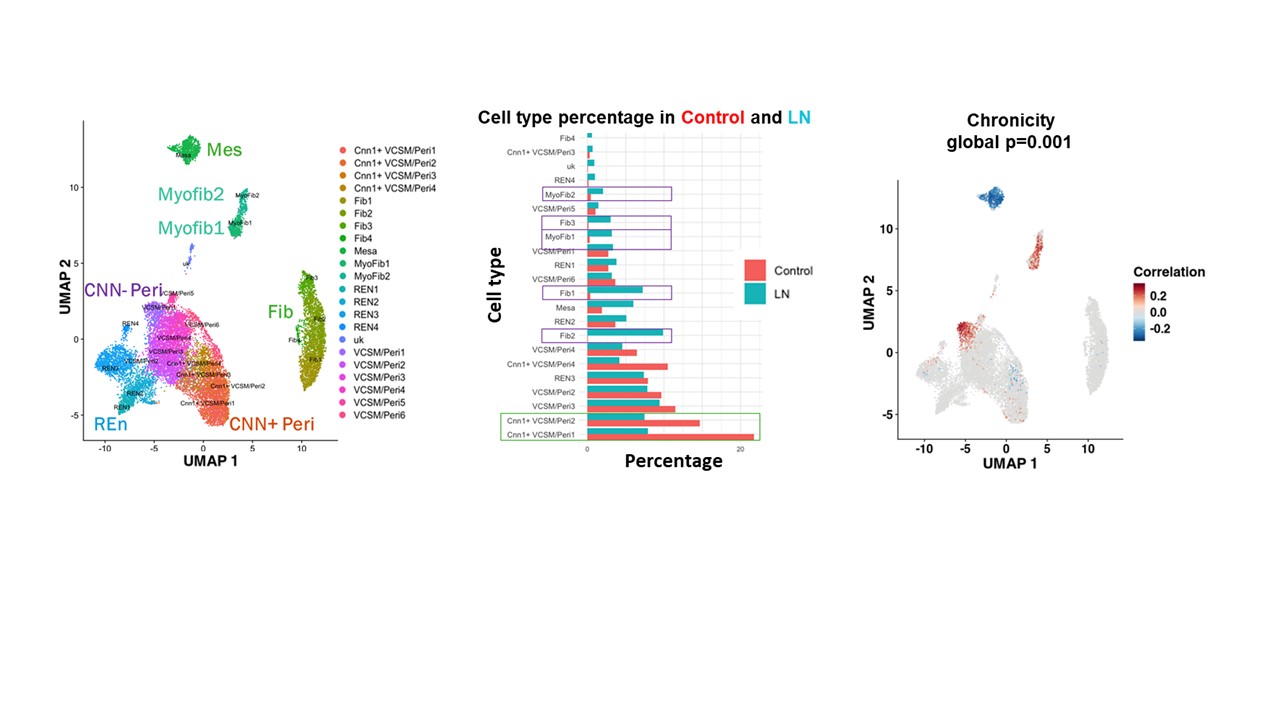 Figure 1: Fibroblast-like PDGFRB+ cell landscape in Lupus Nephritis. A. UMAP of 6 PDGFRB+ subsets, each containing unique subclusters from single-cell RNA-sequencing of stromal cells from 156 LN patients and 30 live donor controls. B. A bar graph of each subcluster as a percentage of total PDGRB+ stromal cells in LN patients (Blue) and controls (red), highlighting subclusters that increased in frequency during LN (Purple box; Fib1-3, Myofib 1-2) and subclusters that decreased (Green box; CNN1+ VSCM/Per1-2). C. Covarying Neighborhood Analysis (CNA) on LN patients projecting onto the PDGFRB+ stromal cells showing association with chronicity. Red indicates a significant positive clinical correlation with the cell subset, while blue indicates a significant negative correlation. The false detection rate is less than 0.1.
Figure 1: Fibroblast-like PDGFRB+ cell landscape in Lupus Nephritis. A. UMAP of 6 PDGFRB+ subsets, each containing unique subclusters from single-cell RNA-sequencing of stromal cells from 156 LN patients and 30 live donor controls. B. A bar graph of each subcluster as a percentage of total PDGRB+ stromal cells in LN patients (Blue) and controls (red), highlighting subclusters that increased in frequency during LN (Purple box; Fib1-3, Myofib 1-2) and subclusters that decreased (Green box; CNN1+ VSCM/Per1-2). C. Covarying Neighborhood Analysis (CNA) on LN patients projecting onto the PDGFRB+ stromal cells showing association with chronicity. Red indicates a significant positive clinical correlation with the cell subset, while blue indicates a significant negative correlation. The false detection rate is less than 0.1.
.jpg) Figure 2: Spatial transcriptomic representation of a glomerulus from a LN patient renal biopsy. A. An overview of the area of interest (Top) with damage-associated macrophage transcripts GPNMP, FABP5, and CSF1R are highlighted. Magnification of the boxed area (bottom) shows GPNMP+ and FABP5+ macrophages localizing in the glomeruli and peri-glomeruli areas (white arrows) compared to macrophages expressing CSF1R alone in the interstitium B. Image of the same area (Top) with Myofib2 transcripts ACTA2, ITGA3, and VCAN highlighted. Magnification of the boxed area (bottom) shows Myofib2, located in the glomerulus and in the crescent (C) (white arrows). V indicates an Acta2+ blood vessel. C. Image of the same area (Top) with Myofib1 transcripts PDFGRA and C7 highlighted. Magnification of the boxed area (bottom) details Myofib1, which are predominantly located in the interstitium (white arrows), separate from Myofib2.
Figure 2: Spatial transcriptomic representation of a glomerulus from a LN patient renal biopsy. A. An overview of the area of interest (Top) with damage-associated macrophage transcripts GPNMP, FABP5, and CSF1R are highlighted. Magnification of the boxed area (bottom) shows GPNMP+ and FABP5+ macrophages localizing in the glomeruli and peri-glomeruli areas (white arrows) compared to macrophages expressing CSF1R alone in the interstitium B. Image of the same area (Top) with Myofib2 transcripts ACTA2, ITGA3, and VCAN highlighted. Magnification of the boxed area (bottom) shows Myofib2, located in the glomerulus and in the crescent (C) (white arrows). V indicates an Acta2+ blood vessel. C. Image of the same area (Top) with Myofib1 transcripts PDFGRA and C7 highlighted. Magnification of the boxed area (bottom) details Myofib1, which are predominantly located in the interstitium (white arrows), separate from Myofib2.
To cite this abstract in AMA style:
Raparia C, Hoover P, Hacohen N, Arazi A, Davidson A. Single-Cell and Spatial Profiling Reveal Proinflammatory and Profibrotic Fibroblast-Macrophage Niches in Lupus Nephritis [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/single-cell-and-spatial-profiling-reveal-proinflammatory-and-profibrotic-fibroblast-macrophage-niches-in-lupus-nephritis/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/single-cell-and-spatial-profiling-reveal-proinflammatory-and-profibrotic-fibroblast-macrophage-niches-in-lupus-nephritis/
