Session Information
Session Type: ACR Abstract Session
Session Time: 2:30PM-4:00PM
Background/Purpose: Renal infiltration with macrophages and dendritic cells is associated with poor prognosis in humans with lupus nephritis (LN). However, the precise pathogenic and/or protective functions of these cells in the LN context are still unknown.
Methods: To characterize the myeloid subsets infiltrating the LN kidney we performed RNASeq of 4 subsets of isolated renal macrophages and dendritic cells from kidneys of nephritic (classical DCs, CD103 DCs, F480hi macrophages, F4/80lo monocytes) and prenephritic (F4/80hi macrophages only) NZB/W mice and Ly6Clo peripheral monocytes of nephritic mice. Single cell RNASeq was performed on renal CD11b+/CD11c+ cells from 2 nephritic and 3 young NZB/W mice and peripheral blood monocytes from nephritic mice using 10X genomics technology and labeling with hashtag olionucleotides to allow subsequent deconvolution of samples. Doublets and cells with >1 hashtag, < 500 expressed genes and/or >15% mitochondrial content were excluded.
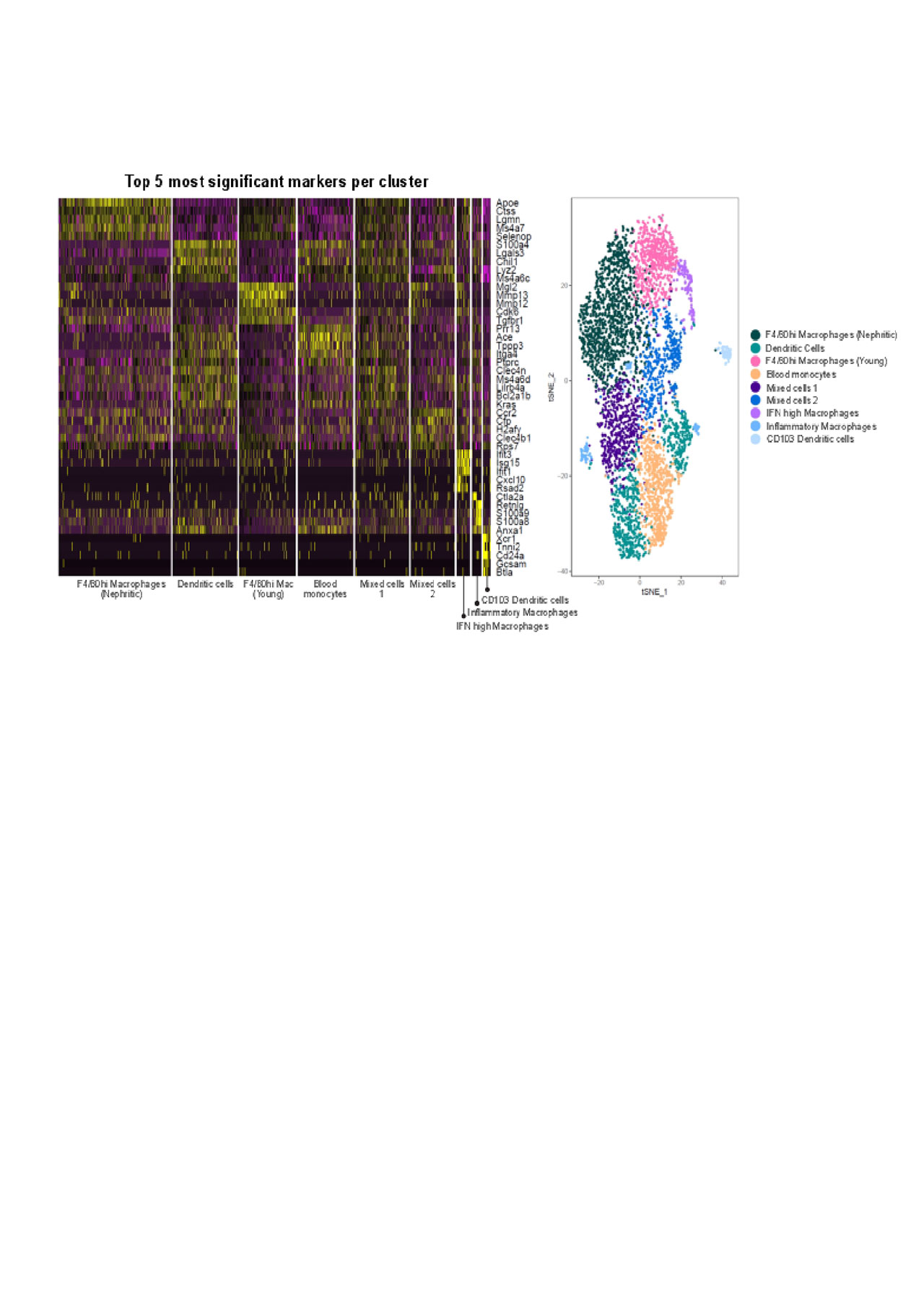
Results: Different cell subpopulations clustered separately by PCA analysis, confirming differences in gene expression between each subset and between young and nephritic mice. Functional analysis of gene expression using GO analysis showed a molecular profile of infiltrating myeloid DCs in nephritic mice that supports lymphoid neogenesis and lymphocyte activation suggesting that these cells help to organize and regulate the inflammatory infiltrates. The profile of F4/80hi macrophages reflects cell metabolism and repair; nevertheless macrophages from nephritic mice had a more inflammatory profile than those from young mice. Single cell analyses were performed on 2096 renal and 1091 blood cells from nephritic and 1449 renal cells from young NZB/W mice. Cluster analysis revealed 9 clusters of myeloid cells of which 7 could be identified as myeloid and CD103 DCs, F4/80hi macrophages (young and nephritic clustered separately), inflammatory macrophages, blood monocytes and a small subset of macrophages with a high IFN signature. The other two subsets comprising < 25% of the cells appeared to contain a mixture of subpopulations including CD209a expressing DCs. Subclustering of the larger clusters revealed that the blood cells and young F4/80hi macrophages were quite homogeneous, whereas F4/80hi macrophages from nephritic mice could be separated into 3 subclusters. Myeloid dendritic cells could be split into 3 subclusters and the two unknown subsets could also each be split into 3 further subclusters. Importantly a significantly higher percentage of IFN high macrophages expressed inflammatory chemokines than the other subsets.
Conclusion:
We found several subsets of renal macrophages in nephritic mice whereas those in young mice were more homogeneous. An interferon high macrophage subset may contribute disproportionately to renal inflammation. Dendritic cells appear to play an important role in lymphoid neogenesis but also displayed considerable heterogeneity. An understanding of the heterogeneity of infiltrating myeloid cell types in LN may allow more precise targeting of those cells that are most likely to be causing renal damage while preserving the protective function of resident cells.
For Anne_ACR abstract_06032019
To cite this abstract in AMA style:
Mishra R, Berthier C, Geiger H, Zhang W, Davidson A. Single Cell Analysis of Renal Myeloid Cells from NZB/WF1 Mice with Lupus Nephritis Reveals Multiple Subsets with Altered Functions [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/single-cell-analysis-of-renal-myeloid-cells-from-nzb-wf1-mice-with-lupus-nephritis-reveals-multiple-subsets-with-altered-functions/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/single-cell-analysis-of-renal-myeloid-cells-from-nzb-wf1-mice-with-lupus-nephritis-reveals-multiple-subsets-with-altered-functions/
