Session Information
Date: Saturday, November 16, 2024
Title: RA – Treatment Poster I
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: The therapeutic equivalence of CT-P47 (a proposed biosimilar to reference tocilizumab [ref-tocilizumab]) was demonstrated in patients with moderate to severe RA through disease activity scores using 28 joints (DAS28) at Weeks 12 and 24 in line with different regulatory requirement. Comparable clinical efficacy and safety up to Week 32 have also been confirmed1. Here, we present the efficacy, pharmacokinetics (PK) and safety of CT-P47 over 1-year, including the outcomes of single transition from ref-tocilizumab to CT-P47.
Methods: 471 moderate-to-severe RA patients who had an inadequate response to ≥1 disease-modifying antirheumatic drugs were randomized (1:1) to receive 8 mg/kg of CT-P47 or ref-tocilizumab intravenously every 4 weeks up to Week 20. Prior to dosing at Week 24, 444 patients were randomized again to either maintain their treatments or switch from ref-tocilizumab to CT-P47, continuing treatment until week 48, followed by a 4-week follow-up period. Efficacy, PK, safety, and immunogenicity were evaluated throughout the study period.
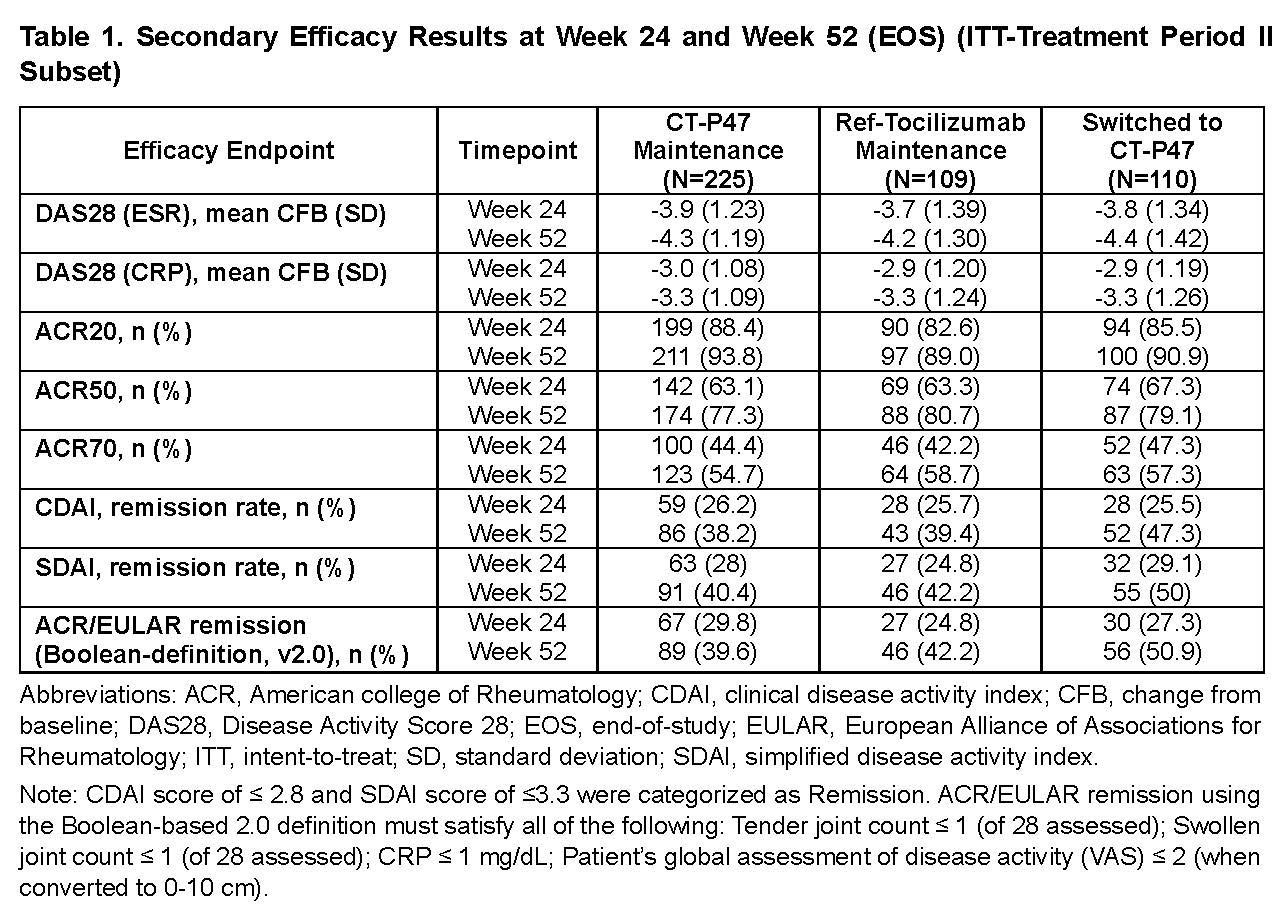
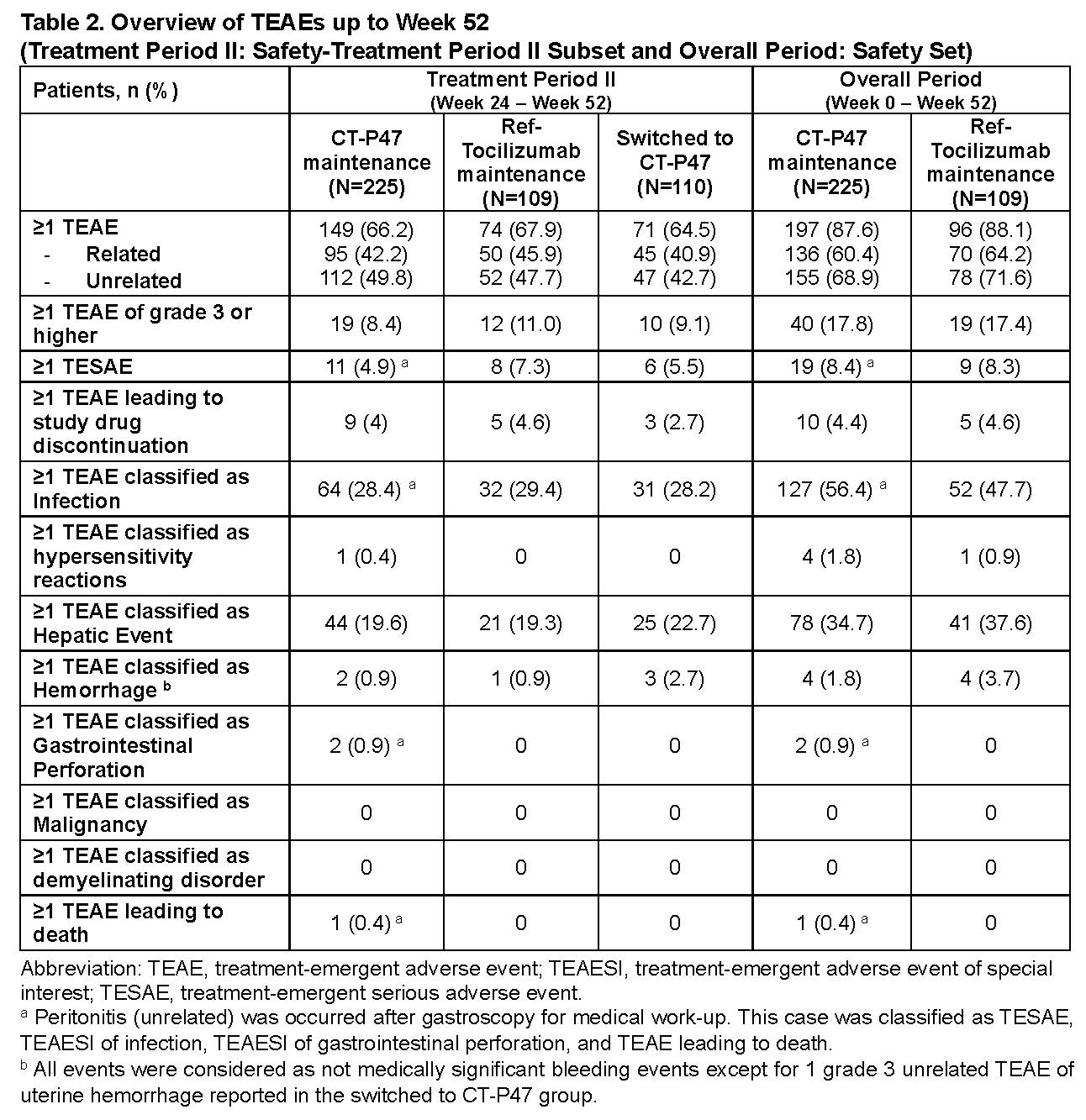
Results: Of 444 patients re-randomized (225 in CT-P47 maintenance, 109 in ref-tocilizumab maintenance, and 110 in Switched to CT-P47), 412 (92.8%) patients completed the treatment and the study (210 in CT-P47 maintenance, 100 in ref-tocilizumab maintenance, and 102 in Switched to CT-P47). The mean change from baseline of DAS28 (ESR) was not affected by transition from ref-tocilizumab to CT-P47 at Week 24, and comparable improvement in clinical activity was observed up to Week 52 (end-of-study [EOS] visit). The rates of ACR20/50/70 and remissions by CDAI, SDAI, and Boolean-definition (v2.0) also showed similar responses among the groups after transition (Table 1). Mean pre-dose serum concentration was similar among the groups up to Week 52 (EOS) (Week 52: CT-P47 Maintenance; 16.73 μg/mL vs ref-tocilizumab Maintenance; 17.41 μg/mL vs Switched to CT-P47; 17.32 μg/mL). The safety profiles from Weeks 24 to 52 (EOS) after transition were generally comparable across the groups. Additionally, those patients who continued to receive CT-P47 or ref-tocilizumab for the entire 52-week period also showed comparable results (Table 2). The proportion of patients who had at least 1 anti-drug antibody positive result at post-treatment after re-randomization was similar among the groups (CT-P47 Maintenance: 4.4% vs. ref-tocilizumab Maintenance: 4.6% vs. Switched to CT-P47: 3.6%), and 1.8% of patients in each group also had at least 1 neutralizing antibody positive result.
Conclusion: The study showed that CT-P47 was well tolerated over 1 year in patients with RA, with comparable efficacy, PK, safety, and immunogenicity to those of ref-tocilizumab. In addition, the efficacy has been sustained after switching from ref-tocilizumab to CT-P47 and no notable safety issue was identified following single transition.
Reference: 1. Smolen, et al. POS0610 Similar Efficacy, Safety, and Immunogenicity of Tocilizumab Biosimilar (CT-P47) and Reference Tocilizumab in Patients with Moderate-to-Severe Active Rheumatoid Arthritis: Week 32 Results from the Phase III Single Transition Study. Ann Rheum Dis. 2024.
To cite this abstract in AMA style:
Burmester G, Trefler J, Racewicz A, Jaworski J, Zielińska A, Krogulec M, Jeka S, Wojciechowski R, Kolossa K, Dudek A, Krajewska-Włodarczyk M, Hrycaj P, Klimiuk P, Kim S, Suh J, Yang G, Kim Y, Jung Y, Hong J, Smolen J. Similar Efficacy, PK, Safety, and Immunogenicity of Tocilizumab Biosimilar (CT-P47) and Reference Tocilizumab in Patients with Moderate-to-Severe Active Rheumatoid Arthritis: Week 52 Results from the Phase III Single Transition Study [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/similar-efficacy-pk-safety-and-immunogenicity-of-tocilizumab-biosimilar-ct-p47-and-reference-tocilizumab-in-patients-with-moderate-to-severe-active-rheumatoid-arthritis-week-52-results-from-the/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/similar-efficacy-pk-safety-and-immunogenicity-of-tocilizumab-biosimilar-ct-p47-and-reference-tocilizumab-in-patients-with-moderate-to-severe-active-rheumatoid-arthritis-week-52-results-from-the/