Session Information
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Sarilumab, as monotherapy or in combination with conventional synthetic DMARDs like methotrexate (MTX) has demonstrated improvement in symptomatic and functional outcomes in patients (pts) with RA. Comparison of efficacy between monotherapy and combination therapy is important due to suboptimal adherence and persistence to MTX in patients with RA and lack of direct comparison studies. Here, we report the results from a post hoc analysis that compared the efficacy of sarilumab monotherapy (MONARCH study; NCT02332590) vs sarilumab and MTX combination therapy (MOBILITY study; NCT01061736).
Methods: MONARCH and MOBILITY were phase III, double-blind randomized controlled studies in pts with RA who showed inadequate responses to or were intolerant to MTX. In MONARCH, pts (enrolled based on 2010 ACR/EULAR criteria) were randomized to receive sarilumab or adalimumab in combination with placebo for 24 weeks. In MOBILITY, pts (enrolled based on 1987 ACR revised classification criteria) were randomized to receive sarilumab or placebo in combination with weekly MTX for 52 weeks. The primary objective of this analysis was to compare the efficacy of sarilumab (200 mg q2w) as monotherapy and in combination with MTX, at Week 24. The endpoints were mean change from baseline in Clinical Disease Activity Index (CDAI), 28-joint Disease Activity using CRP (DAS28-CRP), CRP, hemoglobin (Hb), pain visual analogue scale (VAS) and Functional Assessment of Chronic Illness Therapy (FACIT)-Fatigue. For unadjusted comparisons of efficacy between monotherapy and combination therapy treatment arms, mean change from baseline (95% confidence interval [CI]) at Week 24 in end points was compared between the treatment arms. For adjusted comparisons, continuous change from baseline in endpoints were set as dependent variables and pt baseline characteristics that differed significantly (P< 0.05) between the two trials were set as covariates in mixed-effect model repeated measure (MMRM) models; least square (LS) mean change from baseline (95% CI) at Week 24 in end points was compared between the treatment arms.
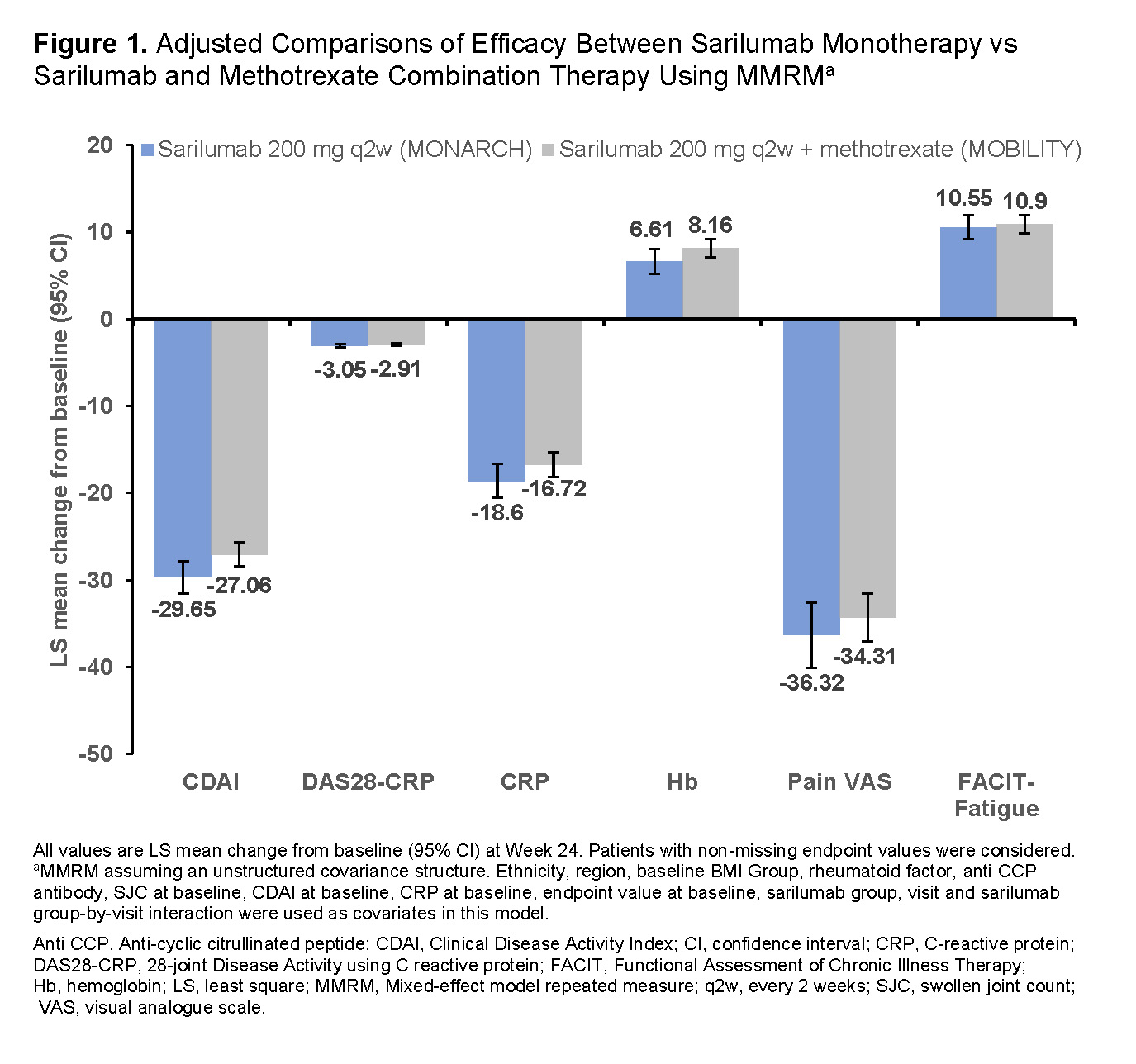
Results: This analysis included 184 pts from the sarilumab monotherapy arm (MONARCH) and 399 pts from the sarilumab plus MTX treatment arm (MOBILITY). Among the baseline characteristics of pts in these two trials, differences (P< 0.05) were observed in ethnicity, region, body mass index group, rheumatoid factor, anti-citrullinated peptide antibodies, swollen joint count, CRP, and clinical disease activity index between the treatment arms (Table 1). After adjusting for these differences in MMRM, LS mean change from baseline at Week 24 for all assessments was similar between the treatment arms with overlapping confidence intervals (Figure 1). Results of unadjusted comparisons were similar to adjusted comparisons (data not shown). The safety profile of sarilumab was previously reported.
Conclusion: Findings from this post hoc analysis of combined data demonstrated that the efficacy of sarilumab as monotherapy was similar to its efficacy as a combination therapy with MTX. These data support previous observations that interleukin (IL)-6R blockade with or without concomitant conventional synthetic DMARDs have similar efficacy.
To cite this abstract in AMA style:
Burmester G, Bykerk V, Buch M, Tanaka Y, Kameda H, Praestgaard A, van Hoogstraten H, Fernandez-Nebro A, Huizinga T. Similar Efficacy of Sarilumab Monotherapy (MONARCH) vs Sarilumab and Methotrexate Combination Therapy (MOBILITY B) in Patients with Rheumatoid Arthritis [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/similar-efficacy-of-sarilumab-monotherapy-monarch-vs-sarilumab-and-methotrexate-combination-therapy-mobility-b-in-patients-with-rheumatoid-arthritis/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/similar-efficacy-of-sarilumab-monotherapy-monarch-vs-sarilumab-and-methotrexate-combination-therapy-mobility-b-in-patients-with-rheumatoid-arthritis/