Session Information
Session Type: Abstract Session
Session Time: 2:00PM-3:30PM
Background/Purpose: High placebo response rates have challenged interpretation of clinical trial results in SLE and may have contributed to failure of some effective treatments. One hypothesis to explain inflated placebo responses is that patients may not have sufficient disease activity at baseline. Most study designs require at entry a score of ≥6 on the SLE Disease Activity Index (SLEDAI). Some have attempted to enrich for more active disease by adding an additional inclusion criterion using BILAG: score of A (severe) in ≥1 organ system and/or B (moderate) in ≥2 organ systems (BILAG restricted studies). However, the impact of this requirement on enrollment and study outcomes, including placebo responses, has not previously been examined.
Methods: Data from all reported phase II & III SLE trials with ≥100 subjects enrolled, at least 24 weeks treatment duration, and SLE responder index (SRI) and/or BILAG-based combined lupus assessment (BICLA) results were analyzed. In studies that did not require BILAG 1A or 2B at entry (BILAG non-restricted studies), the proportion of patients who nevertheless had at least 1 BILAG A or 2B was examined. BILAG restricted trials were compared to non-restricted for meeting primary endpoints, SRI-4, BICLA, placebo responses, and recruitment rates.
Results: Of 40 trials that met the initial search criteria, 3 were excluded from the analysis for not allowing BILAG A at screening (2 trials) and for major changes in entry criteria during study (1 trial). Of the 37 remaining studies (21 phase II and 16 phase III), 18 were BILAG-restricted and 19 non-restricted. In non-restricted studies, the mean percent of patients with at least 1 BILAG A or 2B scores was 62.7%.
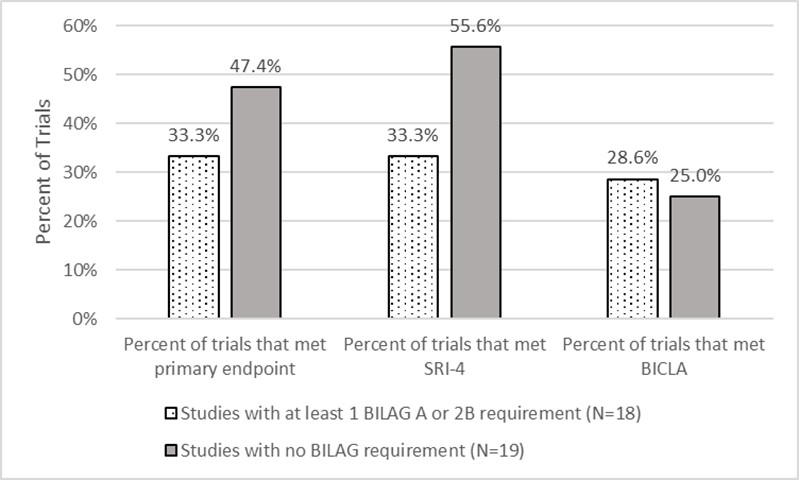
Fifteen of 37 trials (41%) met the primary endpoint, including 6 of 18 (33%) restricted and 9 of 19 (47%) non-restricted studies (Figure 1). Of 36 studies with available SRI-4 data, this endpoint was met by 16 (44%) overall, 6 (33%) of 18 restricted and 10 (56%) of 18 non-restricted studies. Nineteen studies measured BICLA, which was met by 5 (26%) overall, including 4 (29%) of 14 restricted and 1 (25%) of 4 non-restricted.
Mean SRI-4 and BICLA placebo responses in all the studies were 43% and 36%: 44.3% and 36% in restricted studies, 41.7% and 35.9% in non-restricted studies.
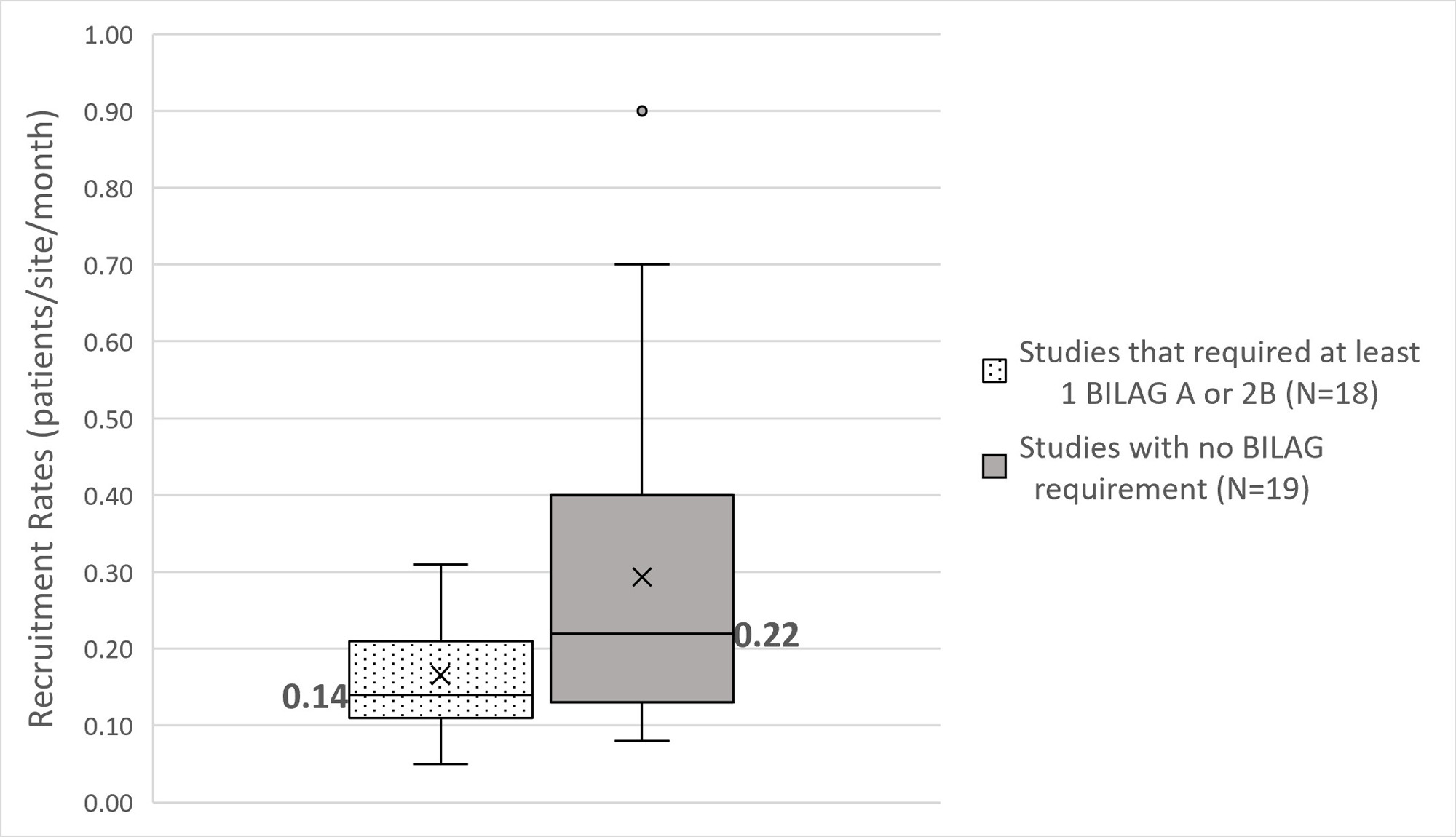
Mean and median recruitment rates for all studies combined were 0.23 and 0.17 patients/site/month (p/s/m). BILAG restricted trials enrolled significantly slower than non-restricted with mean/median 0.17/0.14 p/s/m versus 0.29/0.22 p/s/m (p=0.047, Mann-Whitney test, Figure 2).
Conclusion: Phase II and III SLE studies that require at least 1 BILAG A or 2B at screening are not more likely to meet their primary endpoint. BILAG-restriction does not decrease placebo responses. Furthermore, this entry criterion decreases the eligible patient pool by almost 40% significantly slowing recruitment rates.
To cite this abstract in AMA style:
Olech E, Merrill J. Should SLE Patients Entering Clinical Trials Be Required to Have at Least One BILAG a And/or Two BILAG B Scores? [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/should-sle-patients-entering-clinical-trials-be-required-to-have-at-least-one-bilag-a-and-or-two-bilag-b-scores/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/should-sle-patients-entering-clinical-trials-be-required-to-have-at-least-one-bilag-a-and-or-two-bilag-b-scores/