Session Information
Session Type: Poster Session B
Session Time: 9:00AM-10:30AM
Background/Purpose: Dedicated data on COVID-19 vaccine-related adverse events (ADEs) in patients with systemic sclerosis (SSc) is limited. Therefore, we aimed to compare all patient-reported ADEs post-COVID-19 vaccination in patients with SSc with those in healthy controls (HCs) or non-SSc autoimmune rheumatic diseases (NS-AIRDs).
Methods: Data from the international patient-reported online survey on COVID-19 Vaccination in Autoimmune Diseases (COVAD) study conducted in 2021 by more than 110 collaborators across 94 countries was analyzed. Major and minor ADEs following receipt of COVID-19 vaccine were compared between patients with SSc, those with NS-AIRDs, or with other autoimmune diseases (AIDs) and HCs. Multivariable regression analysis adjusting for age, gender, ethnicity, vaccine type, and immunosuppressants received were performed.
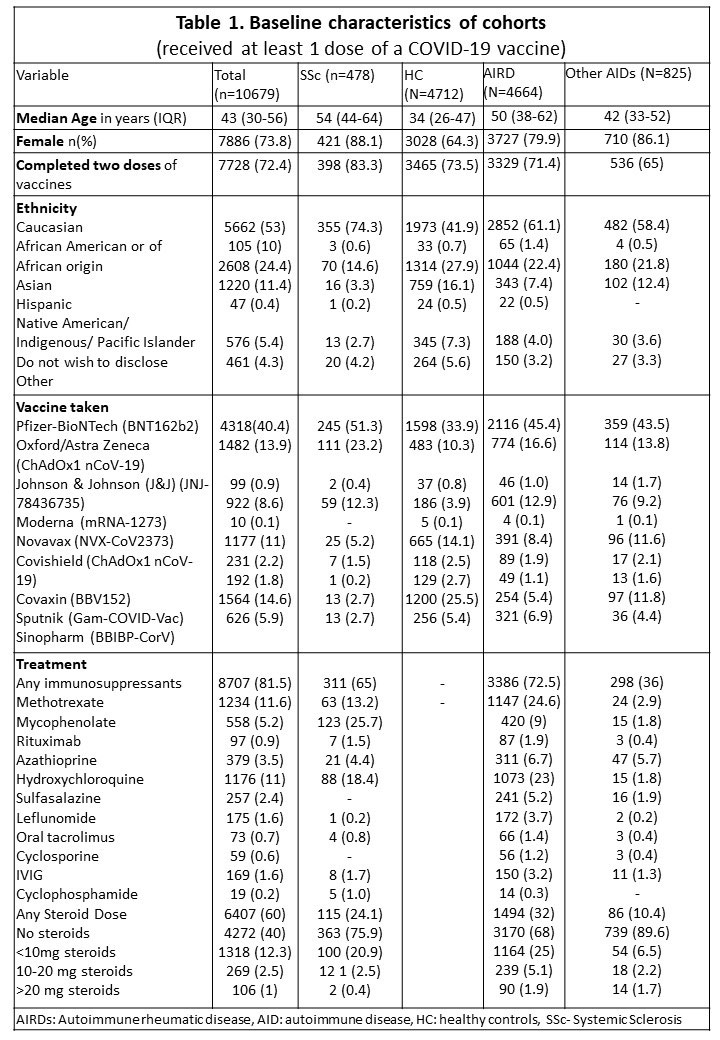
Results: Of the 10,679 respondents (Table 1), there were 478 SSc patients (4.4%) with a mean (SD) age 53.8 (13.3) years, comprising 88% females. At the time of vaccination, 65% were on immunosuppressants and 24% on glucocorticoids. The most common immunosuppressant was mycophenolate mofetil (25.7%).
Among the SSc patients, 83% had completed two vaccine doses. Pfizer-BioNTech (BNT162b2) (51%) and Oxford/AstraZeneca (ChAdOx1 nCoV-19) (23%) were the most common vaccines received. Vaccination related ADEs (Table 2) were reported in 81.2% of SSc patients (81.2%-minor and 3.3% -major ADEs). Patients on hydroxychloroquine reported lower fatigue [OR 0.4 (0.2-0.8)].
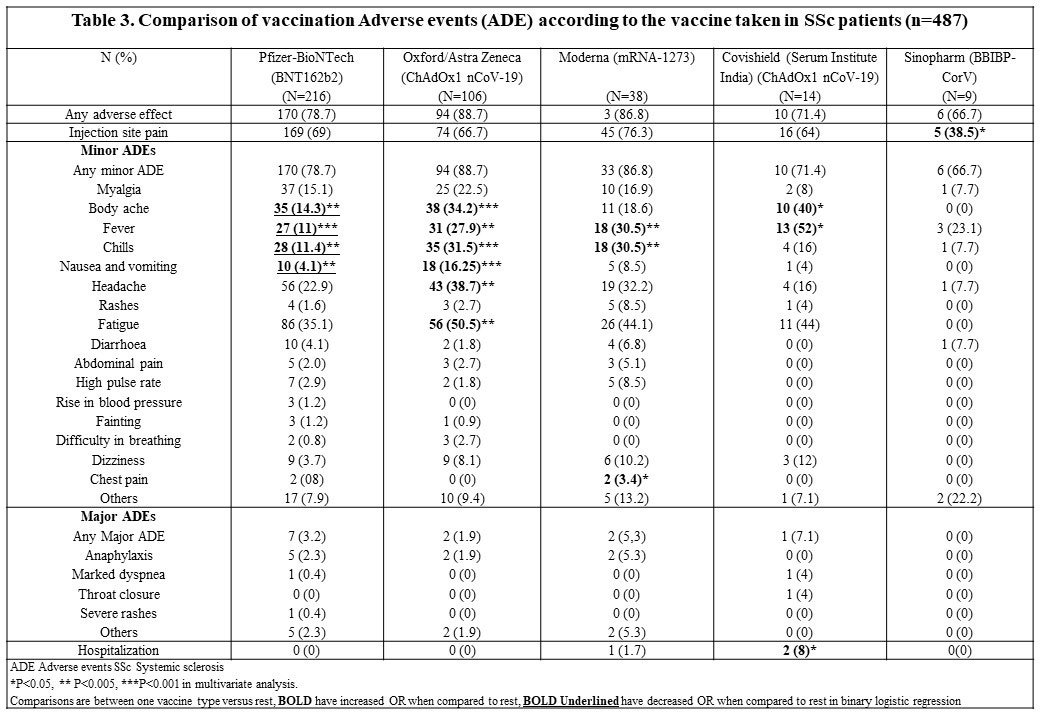
Major ADEs were no different across different vaccine types (Table 3). Pfizer-BioNTech (BNT162b2) recipients reported lower body ache, fever, chills, nausea, and vomiting [OR ranging 0.2-0.4]. Oxford/AstraZeneca (ChAdOx1 nCoV-19) recipients reported higher body ache, fever, chills, nausea vomiting, headache, and fatigue [OR ranging 2.0-5.1]. Moderna (mRNA-1273) recipients reported higher fever, chills, chest pain [OR ranging 2.6-8.9]. Covishield (Serum Institute India) (ChAdOx1 nCoV-19) recipients reported higher body ache, fever, and hospitalization [OR ranging 3.4-13.5]. Sinopharm (BBIBP-CorV) recipients reported lower injection site pain [OR 0.2 (0.06-0.7)].
When compared to HCs and other AIDs, SSc patients reported similar ADEs. When compared to other AIRDs, SSc patients reported higher chills [OR 1.3 (1.0-1.7)] and fatigue [OR 1.3 (1.0-1.6)]. There was no difference in reported hospitalization rates among SSc versus. other AIRDs, other AIDs, and HCs following vaccination.
Conclusion: Despite different vaccines administered worldwide, COVID-19 vaccines were overall largely safe in SSc patients, and vaccine-induced ADEs in SSc were similar to HCs. Pfizer-BioNTech (BNT162b2) recipients reported lower ADE compared to other vaccines.
To cite this abstract in AMA style:
Thakare D, R N, Pauling J, Ahmed S, Wincup C, Del Papa N, Sambataro G, Atzeni F, Govoni M, PARISI S, Bartoloni Bocci E, Sebastiani G, Fusaro E, Sebastiani M, Quartuccio L, Franceschini F, Paolo Sainaghi P, Orsolini G, De Angelis R, Giovanna Danielli M, Venerito V, Sen P, Kim M, Gracia-Ramos A, Yoshida A, Lilleker J, Agarwal V, Kardes S, Day J, Milchert M, Joshi M, Gheita T, Salim B, Parodis I, O’Callaghan A, Nikiphorou E, Chatterjee T, Tan A, Nune A, Cavagna L, Shinjo S, Ziade N, Knitza J, Chinoy H, Distler O, Kuwana M, Aggarwal R, Gupta L, Agarwal V, Makol A. Short-term Safety of COVID-19 Vaccination in Systemic Sclerosis Patients: Report from a Global Patient-Reported E-survey [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/short-term-safety-of-covid-19-vaccination-in-systemic-sclerosis-patients-report-from-a-global-patient-reported-e-survey/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/short-term-safety-of-covid-19-vaccination-in-systemic-sclerosis-patients-report-from-a-global-patient-reported-e-survey/