Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose: The objective of this study was to assess the short-term benefit of etanercept (ETN) + methotrexate (MTX) vs. various disease-modifying anti-rheumatic drugs (DMARDs; hydroxychloroquine [HCQ], leflunomide [LEF], or sulfasalazine [SSZ]) + MTX in subjects with established rheumatoid arthritis (RA).
Methods: Data from subjects with moderate-to-severe RA and an inadequate response to MTX were pooled from the APPEAL1 (ETN 25 mg twice weekly + MTX or DMARD + MTX) and LatinRA2, 3 (ETN 50mg once weekly + MTX or DMARD + MTX) studies. At week 16, proportions of subjects for each pair of treatments were compared using Fisher’s exact test for the following endpoints: American College of Rheumatology (ACR) 20, 50 and 70 responses, disease activity score in 28 joints (DAS28, both C-reactive protein [CRP; DAS28-CRP] and erythrocyte sedimentation rate [ESR; DAS28-ESR] methods) low disease activity (LDA; DAS28-CRP or DAS28-ESR ≤3.2), remission (DAS28-CRP or DAS28-ESR <2.6), Clinical Disease Activity Index (CDAI) of LDA (CDAI ≤10), CDAI remission (CDAI <2.8), and a Health Assessment Questionnaire (HAQ) score of ≤0.5.
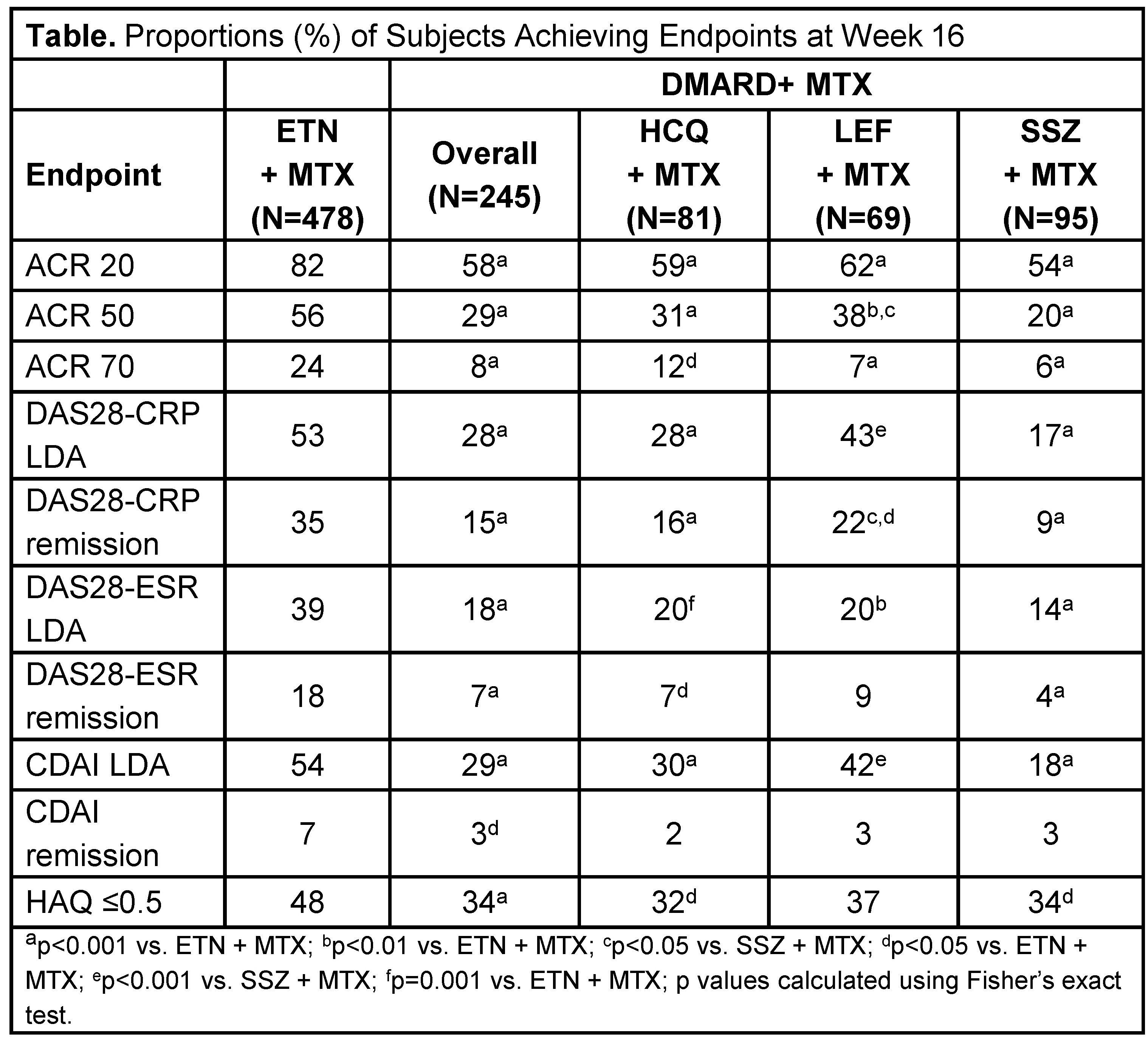
Results: 478 subjects received ETN + MTX and 245 subjects received a DMARD + MTX (HCQ + MTX, n=81; LEF + MTX, n=69; SSZ + MTX, n=95). Baseline demographics were similar between the 2 treatment groups, with a mean age of 48.5 years (standard deviation [SD], 11.7; p=0.983) and disease duration of 7.6 years (SD, 7.5; p=0.437). At week 16, significantly more subjects receiving ETN + MTX achieved ACR 20/50/70, DAS28-CRP LDA and remission, DAS28-ESR LDA and remission, CDAI LDA and remission, and HAQ ≤0.5 compared with subjects on DMARDs + MTX (Table). Significantly greater proportions of subjects in the ETN + MTX group vs. the HCQ + MTX, LEF + MTX, and SSZ + MTX groups reached these endpoints with the exception of CDAI remission for all DMARD types and DAS28-CRP LDA, DAS28-ESR remission, CDAI LDA, and HAQ ≤0.5 in the LEF + MTX group. In addition, significantly more subjects on LEF + MTX achieved ACR50, DAS28-CRP LDA, DAS28-CRP remission, and CDAI LDA than subjects on SSZ + MTX.
Conclusion: Combination ETN + MTX was more effective in treating subjects with established moderate-to-severe RA regardless of the DMARD combination (HCQ, LEF, or SSZ) + MTX. LEF + MTX and HCQ + MTX had some greater benefits over SSZ + MTX at 16 weeks.
References
1. Kim HY et al. Int J Rheum Dis 2012;15:188-96.
2. Machado D et al. Pan American League of Associations for Rheumatology 2012; Poster.
3. Javier R et al. Pan American League of Associations for Rheumatology 2012; Poster.
Disclosure:
R. Fleischmann,
Research grants: Genentech Inc, Roche, Abbott, Amgen, UCB, Pfizer Inc, BMS, Lilly, Sanofi Aventis, Lexicon, MSD, Novartis, BiogenIdec, Astellas, Astra-Zeneca, Janssen,
2,
Roche, Abbott, Amgen, UCB, Pfizer, BMS, Lilly, Sanofi Aventis, Lexicon, Novartis, Astellas, Astra-Zeneca, Janssen, HGS,
5;
A. S. Koenig,
Pfizer Inc,
3;
A. Szumski,
None;
H. Nab,
Pfizer Inc,
1,
Pfizer Inc,
3;
L. Marshall,
Pfizer Inc,
3;
E. Bananis,
Pfizer Inc,
3.
« Back to 2012 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/short-term-efficacy-of-etanercept-plus-methotrexate-vs-various-disease-modifying-anti-rheumatic-combinations-with-methotrexate-in-established-rheumatoid-arthritis/