Session Information
Date: Sunday, November 13, 2022
Title: Pediatric Rheumatology – Clinical Poster II: Connective Tissue Disease
Session Type: Poster Session C
Session Time: 1:00PM-3:00PM
Background/Purpose: To evaluate the short-term efficacy and safety of baricitinib in children withrefractory and/or severe juvenile dermatomyositis (rsJDM) in a real-world setting.
Methods: A monocentric retrospective real-world studyincludingtwenty children with rsJDM was conducted. They were all treated bybaricitinib for 12 to 24 weeks combined with corticosteroids (CS) and/or immunosuppressive agents. Clinical and laboratory data at the baseline (week 0) and week 4, 12, and 24 after treatment were analyzed. Skin Disease Activity Score (skin-DAS), Childhood Myositis Assessment Scale (CMAS)and Manual Muscle Testing-8(MMT-8)were evaluated.
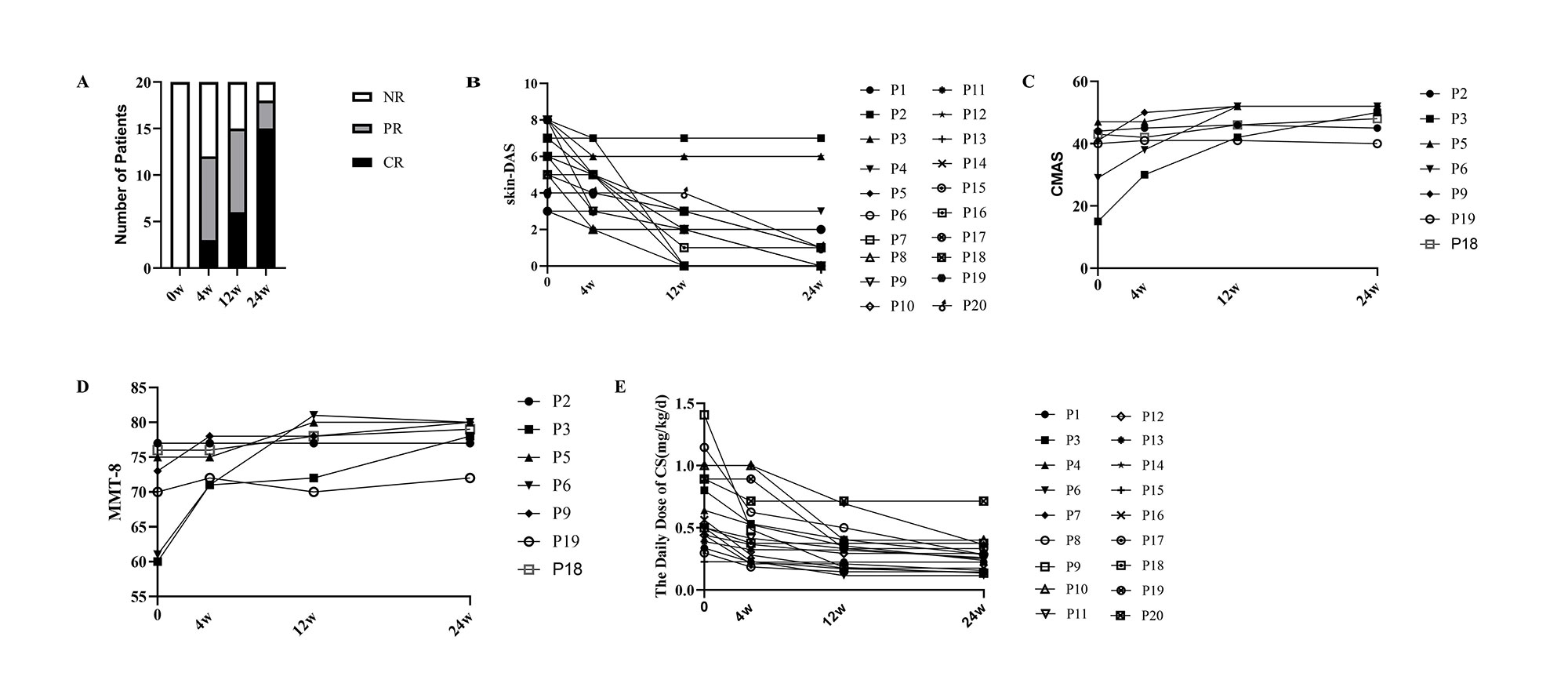
Results: Comparing to baseline, skin rash was improved in 95% patients (19/20) at week 24 with significantly decreased skin-DAS at week 12 [2.0 (0, 3.0) vs 6.0 (4.0, 7.3), p<0.05] and week 24 [1.0(0, 1.0)vs 6.0 (4.0, 7.3), p<0.05]. Comparing to the baseline, CMAS was significantly increased at week 12 [46.0 (42.0, 52.0) vs 41.0(29.0, 44.0), p<0.05 ] and week 24 [52.0 (45.0, 52.0) vs 41.0 (29.0, 44.0), p<0.05] , MMT-8 was significantly increased at week 24 [79.0 (77.0, 80.0) vs 73.0(610, 76.0), p<0.05]. Complete response (CR) and partial response (PR) was achieved in 90% patients (18/20) at week 24 after baricitinib, in which CR was obtained in 15 of 20 patients. The dose of CS was decreased by 37% from baseline 0.53 (0.42,1.0) mg/kg to 0.33 (0.18,0.40) mg/kg at week 12 (P<0.05), and by 49% to 0.27(0.17, 0.37) mg/kg/d at week 24 (P<0.05). There was no significant difference in creatine kinase. No serious side effects had been observed except for one patient with herpes zoster infection.
Conclusion: Baricitinib combined with traditional drugs had efficacy in rsJDM. Add-on therapy of Baricitinib was helpful for tapering the dose of CS.
a: Number of patients achieving CR and/or PR at different follow-up times after baricitinib treatment;b: Decrease of skin-DAS (0–9)at different follow-up times after baricitinib treatment ; c:CMAS (0–52) alteration at seven patients with muscle involvement; d: MMT-8 (0–80) alteration at seven patients with muscle involvement
e: Decrease of the daily dose of CS. rsJDM: refractory and/or severe juvenile dermatomyositis NR:non-response; CR: complete response; PR:partial response;DAS:disease activity score; p:patient;CMAS:childhood myositis assessment scale;MMT:manual muscle testing; CS:corticosteroid
To cite this abstract in AMA style:
wang z, lu m, zheng q. Short-term Efficacy of Baricitinib in Children with Refractory And/or Severe Juvenile Dermatomyositis [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/short-term-efficacy-of-baricitinib-in-children-with-refractory-and-or-severe-juvenile-dermatomyositis/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/short-term-efficacy-of-baricitinib-in-children-with-refractory-and-or-severe-juvenile-dermatomyositis/