Session Information
Date: Sunday, November 13, 2022
Title: SLE – Diagnosis, Manifestations, and Outcomes Poster II: Manifestations
Session Type: Poster Session C
Session Time: 1:00PM-3:00PM
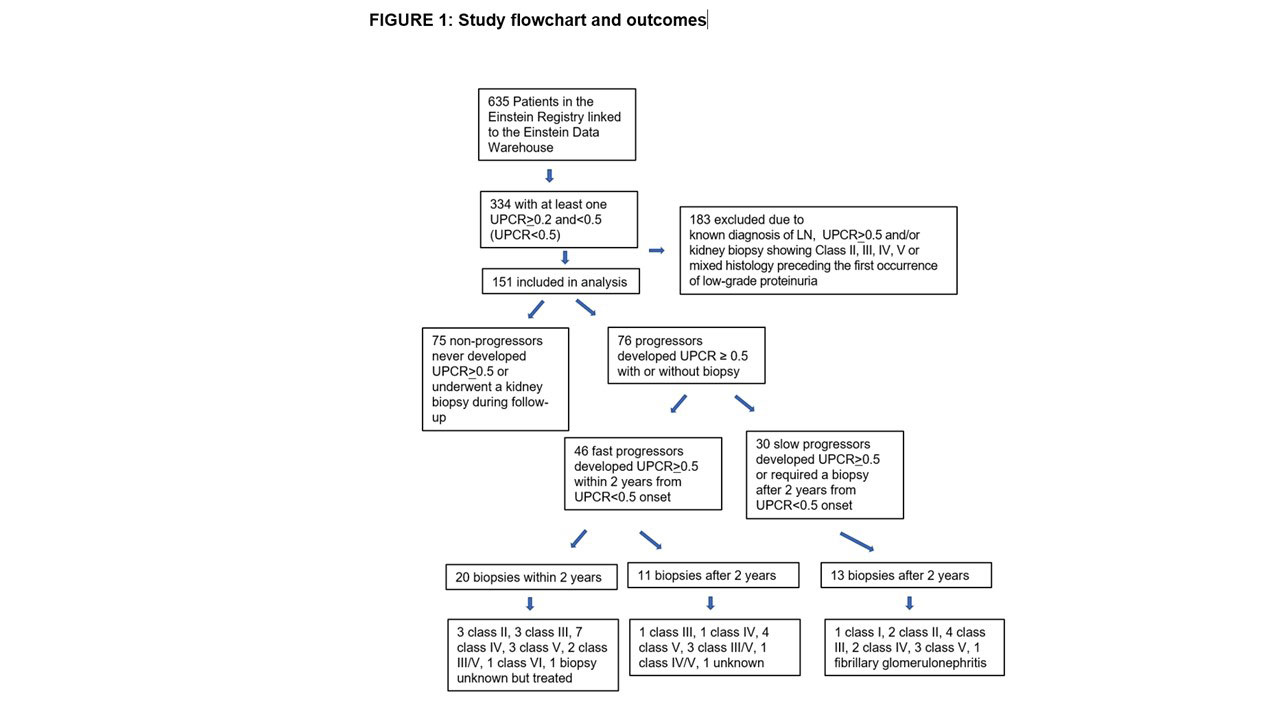
Background/Purpose: Lupus nephritis remains a common cause of morbidity and mortality in systemic lupus erythematosus (SLE). Current guidelines recommend performing a kidney biopsy at urine protein-to-creatinine ratio (UPCR) of ≥0.5. However, cross-sectional studies reported a high prevalence of active histological lupus nephritis lesions, and even chronic scarring, in patients with low-grade proteinuria This study was initiated to assess disease progression in SLE patients with low-grade proteinuria to identify risk factors for progression to overt proteinuria suggestive of clinical lupus nephritis.
Methods: SLE patients with incident UPCR ≥ 0.2 and < 0.5 (UPCR≥ 0.2 &< 0.5) without known lupus nephritis were identified from the Einstein Rheumatic Disease Registry. Patients who developed a random UPCR of ≥ 0.5 with or without biopsy during the follow-up period were defined as “progressors”. Patients who progressed to UPCR>0.5 within two years from developing UPCR≥ 0.2 &< 0.5 were defined as “fast progressors”, a subgroup expected to benefit most from early biopsies and therapeutic interventions.
Results: Among 151 eligible SLE patients with low-grade proteinuria at study entry, 76 (50%) progressed to UPCR≥0.5 of which 44 underwent a clinically indicated biopsy (Figure 1). Sixty-one percent of progressors developed UPCR≥0.5 within 2 years of follow-up. Of the 20 biopsies performed in the first 2 years, 16 had active, treatable lupus nephritis. Low complement and shorter SLE duration at low-grade proteinuria onset most consistently associated with progression to overt proteinuria. Other associated factors included hypertension, diabetes mellitus, younger age and the presence of hematuria.
Conclusion: In this longitudinal cohort, over half of SLE patients with new-onset low-grade proteinuria without a prior diagnosis of lupus nephritis progressed to overt proteinuria and 11 percent had a biopsy consistent with treatable lupus nephritis within 2 years, supporting the role of early biopsy to diagnose and treat lupus nephritis. The data from this study lay the groundwork for future studies of underlying molecular pathways in progression of early lupus nephritis by identifying at risk populations. Early lupus nephritis diagnosis and effective intervention reduce adverse, long-term kidney complications. The ability to prevent injury should result in less overall exposure to toxic medications and better preservation of kidney mass. There is currently a dearth of reliable clinical markers to predict which SLE patients with low grade proteinuria should be biopsied. Understanding the molecular mechanisms activated in the kidney at the earliest stage in high-risk populations will be critical for developing biomarkers of early disease.
To cite this abstract in AMA style:
Wang S, Spielman A, Ginsberg M, Petri M, Rovin B, Buyon J, Broder A. Short- and Long-Term Progression of Kidney Involvement in Systemic Lupus Erythematosus Patients with Low-Grade Proteinuria [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/short-and-long-term-progression-of-kidney-involvement-in-systemic-lupus-erythematosus-patients-with-low-grade-proteinuria/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/short-and-long-term-progression-of-kidney-involvement-in-systemic-lupus-erythematosus-patients-with-low-grade-proteinuria/