Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Although PsA occurs in males and females at similar rates, clinical manifestations and outcomes differ between sexes. Findings from real-world evidence (RWE) studies have found that females tend to have higher disease activity and functional impairment than males despite generally lower rates of radiographic joint damage and milder psoriasis (PsO) symptoms1-5. Despite observed sex-specific differences in PsA, few randomized controlled trials (RCTs) report sex-disaggregated results6. Here, we describe baseline (BL) patient (pt) and disease profiles by sex in a large, pooled cohort of clinical trial participants with active PsA.
Methods: Post hoc analyses included PsA pts from 3 Phase 3 RCTs for guselkumab (GUS): 381 pts from DISCOVER-1 (NCT03162796), 739 from DISCOVER-2 (NCT03158285), and 285 from COSMOS (NCT03796858). BL pt characteristics were compared between sexes using a 2-sample t-test for continuous variables and a chi-square test for categorical variables (see Table 1).
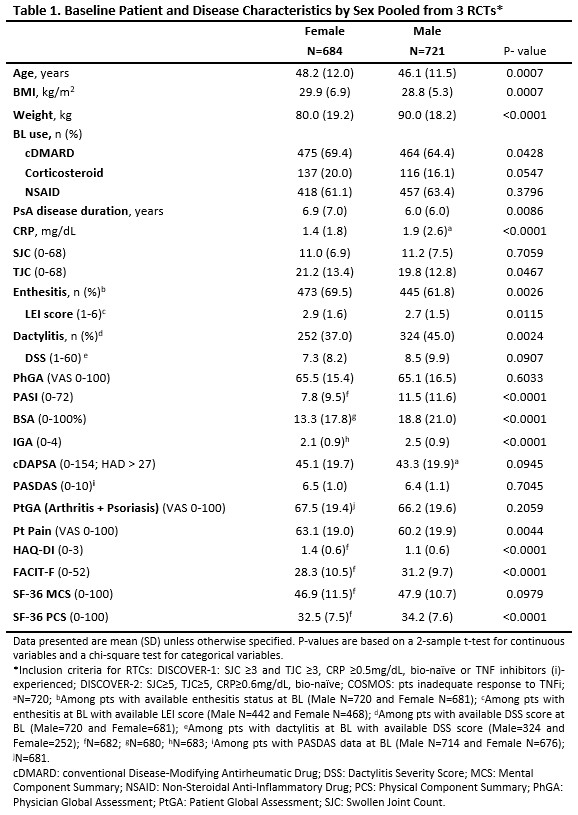
Results: Among 1405 pts with PsA, 48.7% were female. At BL, female pts were older than males (mean: 48.2 vs 46.1y; p=0.0007), had longer PsA disease duration (6.9 vs 6.0y; p=0.0086) and a higher BMI (29.9 vs 28.8 kg/m2; p=0.0007; Table 1). Females had significantly milder PsO at BL than males (mean Psoriasis Area Severity Index [PASI]: 7.8 vs 11.5; body surface area [BSA]: 13.3 vs 18.8%; Investigator’s Global Assessment [IGA]: 2.1 vs 2.5; p< 0.0001). No difference was observed in BL composite measures of disease activity (Clinical Disease Activity Index for PsA [cDAPSA] and PsA Disease Activity Score [PASDAS]); however, several individual components showed differences between females and males, including lower BL CRP levels (mean: 1.4 vs 1.9 mg/dL; p< 0.0001) and higher tender joint count (TJC: 21.2 vs 19.8; p=0.0467) and Leeds Enthesitis Index (LEI) score (2.9 vs 2.7; p=0.0115). Additionally, enthesitis was more prevalent in females (69.5 vs 61.8%; p=0.0026), while dactylitis was more common in males (45.0 vs 37.0%; p=0.0024). Females also reported significantly worse pt-reported outcomes than males at BL, with higher levels of fatigue (mean Functional Assessment of Chronic Illness Therapy-Fatigue [FACIT-F]: 28.3 vs 31.2; p< 0.0001), functional impairment (mean HAQ–Disability Index [DI]: 1.4 vs 1.1; 36‐item Short Form health survey physical component summary [SF-36 PCS]: 32.5 vs 34.2; p< 0.0001), and pain (mean: 63.1 vs 60.2; p=0.0044).
Conclusion: Sex differences in pts with highly active PsA from 3 Phase 3 trials were consistent with findings from RWE cohorts. Female PsA pts exhibited less severe PsO, higher BMI, longer PsA duration, and reported a greater impact of PsA on quality of life. These findings highlight the importance of considering biological sex and associated clinical features in the management of PsA, including the evaluation of response to treatment (see Eder L, et al. ACR 2024; AB1840951).
References:
- Gossec. J Rheumatol 2023;50:192-6
- Duruöz. Joint Bone Spine 2021;88:105177
- Nas. Mod Rheumatol 2017;27:345-9
- Eder. Ann Rheum Dis 2013;72:578-82
- Kojanova. Dermatol Ther 2021;34:e14849
- Eder. Lancet Rheum 2023; 5:e716-27
To cite this abstract in AMA style:
Coates L, Selmi C, Mease P, Ogdie A, Nantel F, Lavie F, Sharaf M, Adelakun O, Rampakakis E, Pina Vegas L, Eder L. Sex-Related Differences in Baseline Patient and Disease Characteristics: Post Hoc Analyses of Three Phase 3, Randomized, Double-blind, Placebo-Controlled Studies in Patients with Active Psoriatic Arthritis [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/sex-related-differences-in-baseline-patient-and-disease-characteristics-post-hoc-analyses-of-three-phase-3-randomized-double-blind-placebo-controlled-studies-in-patients-with-active-psoriatic-arth/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/sex-related-differences-in-baseline-patient-and-disease-characteristics-post-hoc-analyses-of-three-phase-3-randomized-double-blind-placebo-controlled-studies-in-patients-with-active-psoriatic-arth/