Session Information
Session Type: Poster Session D
Session Time: 1:00PM-3:00PM
Background/Purpose: Psoriatic arthritis (PsA) is a progressive, joint-degenerative inflammatory disease with cartilage destruction, bone erosion and growth, and synovitis. Extracellular matrix (ECM) remodeling is also observed in skin lesions in PsA patients (pts). Guselkumab (GUS), an interleukin-23p19-subunit monoclonal antibody, demonstrated robust efficacy compared with placebo in pts with active PsA in two Phase-3 studies, DISCOVER-1 & DISCOVER-21,2. GUS also reduced serum ECM biomarkers shown to be elevated in PsA pts3.
The purpose of this study is to identify treatment responders among PsA pts receiving GUS, using serum biomarkers of collagen formation, degradation, and vimentin degradation.
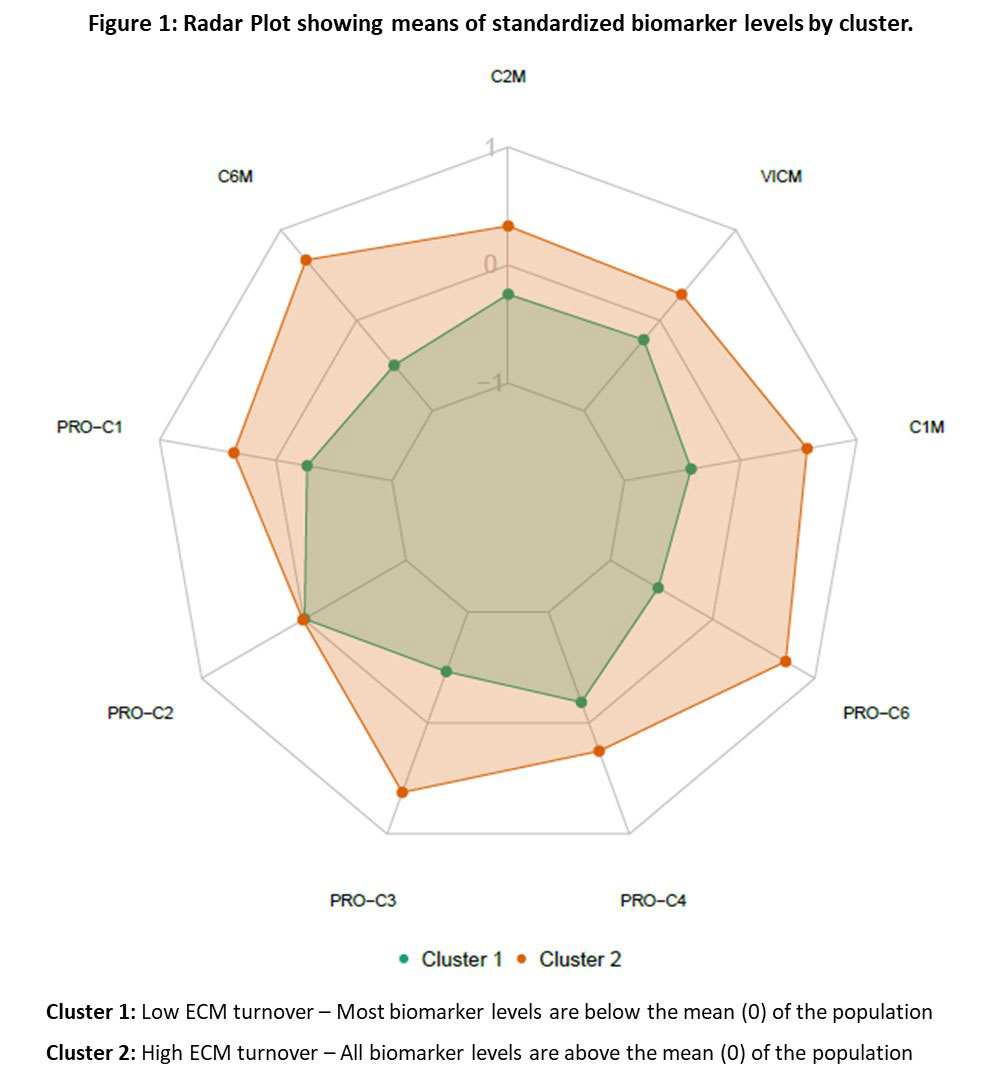
Methods: Available baseline serum ECM biomarker data from patients who consented to participate in the 2-year DISCOVER-2 trial (NCT0315828) were included in the post hoc analysis. Biomarkers of collagen formation (PRO-C2, PRO-C3, PRO-C4, PRO-C6), degradation (C1M, C2M, C6M), and citrullinated and degraded vimentin (VICM) were measured by immunoassays. Baseline ECM biomarker levels from all available pts were log-transformed and standardized by mean centering and scaling by the standard deviation prior to K-means clustering. In GUS-treated pts, between-cluster comparisons for major demographic and clinical parameters were performed using Mann-Whitney or chi-squared tests. Relationships between observed clinical response (ACR20, ACR50, PASI100) at W24, W52, and W100 and baseline levels of biomarkers were investigated. Analysis of PASI response was performed in pts with baseline body surface area (BSA) ≥3% and Investigator’s Global Assessment of psoriasis (IGA) score ≥2.
Results: Two distinct clusters were identified using baseline serum ECM biomarkers from all pts (Figure 1). Pts in Cluster 1 generally had lower levels of ECM biomarkers than did those in Cluster 2. In GUS-treated pts, those in Cluster 2 had higher (all p< 0.05) baseline PASI score (median 7.3 vs 5.1), swollen joint count (12.0 vs 10.0), CRP (2.0 vs 1.0 mg/dL), and PsA-modified vdH-S score (26.5 vs 13.0). Relative to pts in Cluster 1, GUS-treated pts in Cluster 2 showed a greater ACR20 response rate at W24 (72% vs 57%, p=0.039) and W52 (80% vs 66%, p=0.037) and a greater ACR50 response rate at W100 (64% vs 47%, p=0.031), indicating pts with high levels of these biomarkers were more likely achieve an ACR response. For pts with at least mild skin disease at baseline (BSA≥3% and IGA≥2), those in Cluster 1 demonstrated a higher PASI100 response rate at W100 than pts in Cluster 2 (71% vs 54%, p=0.046).
Conclusion: Pts with higher levels of baseline ECM biomarkers (Cluster 2) that correspond with greater peripheral joint inflammation demonstrated higher ACR20 and ACR50 response rates when treated with GUS, while pts with lower levels of these ECM biomarkers (Cluster 1) demonstrated higher rates of complete skin clearance (PASI100). These findings may provide a step towards pt stratification and precision medicine for guiding the use of biologic immunomodulatory treatments in pts with PsA.
References
1. Deodhar, A et al. Lancet. 2020;395:1115–25.
2. Mease, PJ et al. Lancet.2020;395: 1126–36.
3. Schett, G et al. Rheumatol Ther. 2022;1–14.
To cite this abstract in AMA style:
Holm Nielsen S, Bay-Jensen A, Frederiksen P, Karsdal M, Shawi M, Chakravarty S, Kollmeier A, Chen W, Gao S. Serum Extracellular Matrix Biomarkers Identify Response to Guselkumab in Psoriatic Arthritis; Post-hoc Analysis from a Phase 3, Randomized, Double-blind, Placebo-controlled Study Through 2 Years [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/serum-extracellular-matrix-biomarkers-identify-response-to-guselkumab-in-psoriatic-arthritis-post-hoc-analysis-from-a-phase-3-randomized-double-blind-placebo-controlled-study-through-2-years/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/serum-extracellular-matrix-biomarkers-identify-response-to-guselkumab-in-psoriatic-arthritis-post-hoc-analysis-from-a-phase-3-randomized-double-blind-placebo-controlled-study-through-2-years/