Session Information
Date: Monday, November 11, 2019
Title: 4M116: RA – Diagnosis, Manifestations, & Outcomes III: Diagnosis & Prognosis (1884–1889)
Session Type: ACR Abstract Session
Session Time: 4:30PM-6:00PM
Background/Purpose: The ability to accurately predict whether RA patients will flare following DMARD-tapering would open doors in clinical decision-making for achieving drug-free remission (DFR). Calprotectin (S100A8/A9 or MRP8/14), an inflammatory protein released locally by monocytes, is a marker of disease activity in RA1. We hypothesized that circulating calprotectin could indicate residual subclinical inflammation in patients who are in remission on DMARDs, and that patients with high calprotectin levels might thus be more prone to flare after DMARD-tapering. As such, we investigated the value of calprotectin levels as a marker for disease flare after DMARD-tapering.
Methods: We used data from two randomized controlled trials: the IMPROVED2 study and the RETRO3 study. The IMPROVED included early (< 2 years) untreated RA, was steered at disease activity score-remission (DAS44< 1.6), and, for those achieving remission at 8 months, rapidly tapered and stopped methotrexate to attempt drug-free remission. The RETRO randomized patients with long-standing RA in stable remission (DAS44< 2.6) to three arms: continue, taper by 50%, or stop (biological or conventional) DMARDs. Only patients in the taper or stop arms were included for this analysis. Circulating calprotectin at the tapering timepoint was determined using a diagnostic calprotectin kit (Inova Diagnostics, Research use only) in IMPROVED and using an in-house sandwich ELISA4 in RETRO. Logistic regression and area under the receiver operating characteristic curves (AUC) analyses were conducted to evaluate the predictive value of calprotectin for flare (loss of remission) within 12 months of tapering/stopping DMARDs.
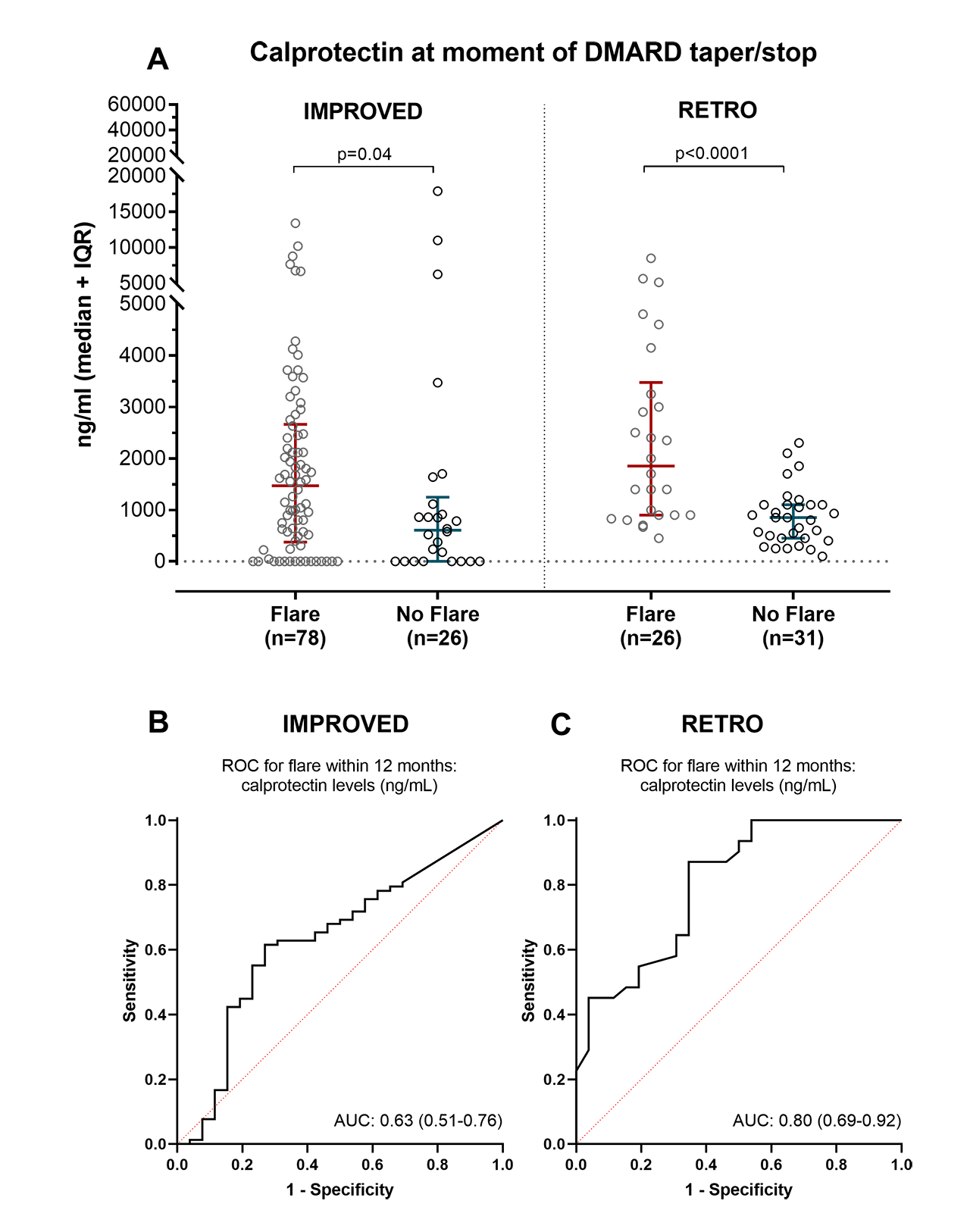
Results: In the IMPROVED and in the RETRO, respectively 104 and 57 patients were eligible to taper/stop DMARDs and had serum available. In both IMPROVED and RETRO, patients that flared within 12 months had higher calprotectin at the moment of DMARD-tapering/stop (Figure 1A). Two-fold higher calprotectin at the moment of DMARD-tapering/stop was associated with an increased risk (odds ratio) of flare of 1.1 (95% CI: 1.0-1.2) in the IMPROVED study and 3.6 (95% CI: 1.8-7.5) in the RETRO study. Correcting for clinical predictors of flare (body mass index, gender, anti-CCP2, and baseline DAS in IMPROVED; age and anti-CCP2 in RETRO) did not greatly alter these estimates: 1.1 (95% CI: 1.0-1.2) in the IMPROVED study and 4.0 (95% CI: 1.8 to 8.9) in the RETRO study. The AUC for predicting flare within 12 months was 0.63 (95% CIs: 0.51-0.76) in the IMPROVED study and 0.80 (95% CIs: 0.69 to 0.92) in the RETRO study (Figure 1B-C).
Conclusion: Circulating calprotectin levels in RA patients in remission on DMARDs are associated with higher risk of disease flare upon DMARD-tapering. Calprotectin’s ability to predict whether RA patients will flare following DMARD-tapering could aid in clinical decision-making for attempting drug tapering and discontinuation.
References:
- doi:10.3899/jrheum.140628
- doi:10.1136/annrheumdis-2017-211375
- doi:10.1136/annrheumdis-2014-206439
- doi:10.1136/annrheumdis-2011-200598
To cite this abstract in AMA style:
de Moel E, Rech J, Mahler M, Roth J, Schouffoer A, Ronday K, Huizinga T, Allaart C, Toes R, Schett G, van der Woude D. Serum Calprotectin Is a Prognostic Marker for Drug-Free Remission in RA [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/serum-calprotectin-is-a-prognostic-marker-for-drug-free-remission-in-ra/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/serum-calprotectin-is-a-prognostic-marker-for-drug-free-remission-in-ra/