Session Information
Date: Saturday, November 6, 2021
Title: SLE – Diagnosis, Manifestations, & Outcomes Poster I: Diagnosis (0323–0356)
Session Type: Poster Session A
Session Time: 8:30AM-10:30AM
Background/Purpose: Galectin-9, interferon-inducible protein-10 (IP-10) and sialoadhesin (SIGLEC-1) are proteins associated with interferon signature, and considered as potential biomarkers reflecting disease activity in patients with systemic lupus erythematosus (SLE). In this study, we aimed to investigate the association of serum and urine levels of galectin-9, IP-10 and SIGLEC-1 with disease activity in patients with SLE.
Methods: Sixty-three patients with active SLE (31 renal and 32 extrarenal) were included in the study. Thirty inactive patients with SLE (15 renal and 15 extrarenal) and 32 healthy volunteers were selected as control groups. Serum (s) and urine (u) levels of galectin-9, IP-10 and SIGLEC-1 were tested using ELISA. Urine levels of biomarkers were normalized by urine creatinine.
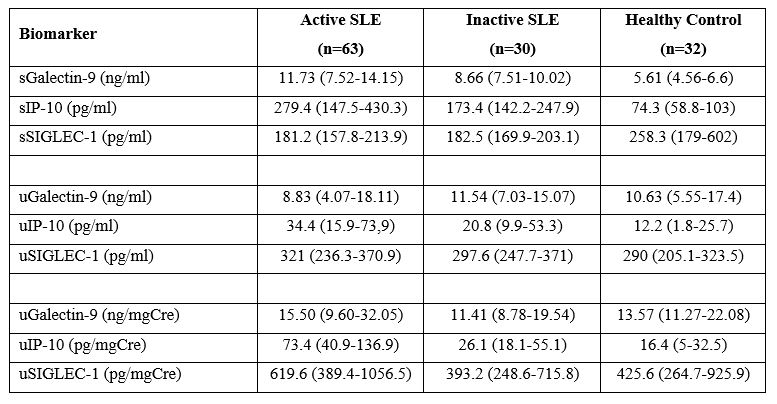
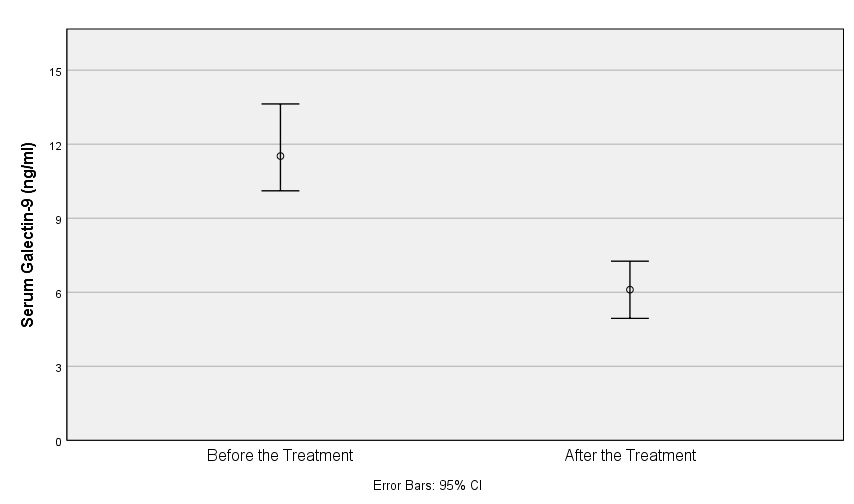
Results: Groups were comparable with regard to sex and age distribution. Of 125 participants, 102 (81.6%) were female and median age was 33 (28-44.5) years. Proliferative lupus nephritis (LN) (class III/III+V and IV/IV+V) were found in 22 patients with active renal SLE (70.9%), while 6 patients (19.3%) had pure class V and 3 (9.7%) had class II LN. Levels of sIP-10, uIP-10, sGalectin-9 and uSIGLEC-1 were significantly higher in the active SLE group compared to the inactive SLE group (sIP-10 p=0.046, uIP-10 p< 0.001, sGalectin-9 p=0.031 and uSIGLEC-1 p=0.006); however, no differences were detected in the comparison of uGalectin-9 and sSIGLEC-1 between the groups (uGalectin-9 p=0.180 and sSIGLEC-1 p=0.699) (Table 1). Serum and urine levels of galectin-9, IP-10 and SIGLEC-1 did not differ between patients with active renal and extrarenal SLE. Levels of sIP-10, uIP-10 and uSIGLEC-1 were correlated with SLE Disease Activity Index (SLEDAI). Serum and urine levels of all biomarkers were re-tested in 41 of 63 patients (65%) with active SLE after a median treatment of 8 (5-22.5) months. At the time of the second tests, there was a significant decrease in disease activity as measured by SLEDAI [2 (0-4)] compared to the time of the first tests [10 (6-15.5)]. Comparison of sGalectin-9 levels between the serum at the time of active disease and remission showed a very significant decline (p< 0.001) as shown in Figure 1. uGalectin-9, sIP-10 and uSIGLEC-1 also decreased after treatment; however, the difference was not statistically significant.
Conclusion: sIP-10, uIP-10, sGalectin-9 and uSIGLEC-1 are associated with disease activity in SLE. None is able to discriminate active renal from active extrarenal disease. sGalectin-9 may be a valuable biomarker to monitor response after treatment for active disease (Funded by Scientific Research Projects Coordination Unit of Istanbul University. Project number: TSA-2019-34218).
To cite this abstract in AMA style:
Mirioglu S, Cinar S, Uludag O, Gurel E, Varelci S, Ozluk Y, Kilicaslan I, Yalcinkaya Y, Yazici H, Gül A, Inanc M, Esen B. Serum and Urine Galectin-9, IP-10 and SIGLEC-1 as Biomarkers of Disease Activity in Patients with Systemic Lupus Erythematosus [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/serum-and-urine-galectin-9-ip-10-and-siglec-1-as-biomarkers-of-disease-activity-in-patients-with-systemic-lupus-erythematosus/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/serum-and-urine-galectin-9-ip-10-and-siglec-1-as-biomarkers-of-disease-activity-in-patients-with-systemic-lupus-erythematosus/