Session Information
Session Type: Poster Session (Sunday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Patient-reported outcomes (PROs) measures are a key component of the care of patients with systemic lupus erythematosus (SLE). The Patient Reported Outcomes Measurement Information System (PROMIS) computerized adaptive test (CAT) is a recently developed PRO instrument that has not yet been well studied in SLE. This study seeks to examine the sensitivity to change of PROMIS CAT compared to patient-reported anchors within a cohort of adult SLE patients.
Methods: All consecutive adult (≥18 years old) English-speaking patients with SLE and visiting the Toronto Lupus Clinic between July-September 2018 were approached to participate. Patients completed PROMIS CAT during their clinical visit assessing 14 domains. 89 patients completed PROMIS CAT at baseline and at 3-month follow-up. At 3 months, the generic anchor question “Compared to when you started the study, how have you been during the last 48 hours? (responses: same, worse, )” was asked to identify two subsets of patients, those with improved vs worsened symptoms at 3 months. Similar domain-specific anchor questions were asked at 3 months, with responses graded from -7 (greatest worsening) to +7 (greatest improvement), and 0 representing no change. For domain-specific anchors, patients were deemed to have improved if they graded >1, and worsened if < -1. Responsiveness was evaluated using standardized response means (SRM), with higher SRM values reflecting greater sensitivity to change (0.3-0.5 low, 0.5-0.8 moderate, >0.8 high). SRMs for each domain were determined with respect to the generic as well as domain-specific anchors. We hypothesized that domains demonstrate worsening and improvement in concordance with the anchors.
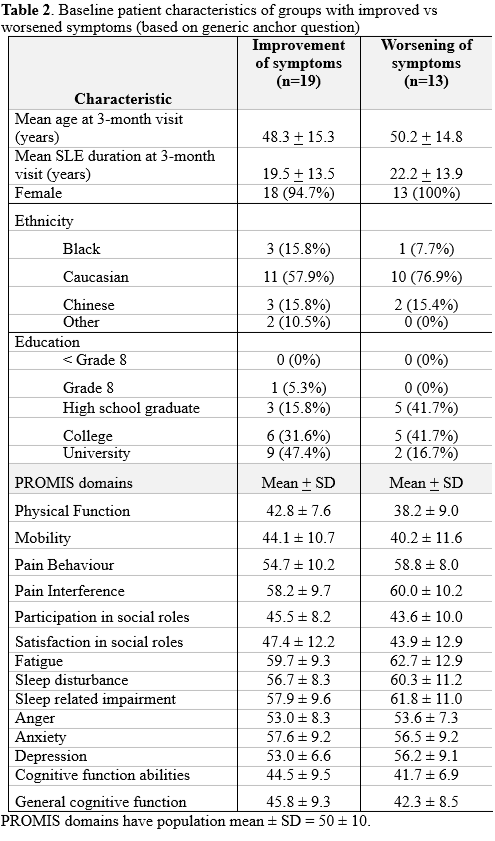
Results: Patient characteristics are in Table 1. Table 2 contains the baseline characteristics of the subsets of patients with improvement and worsening at follow-up.
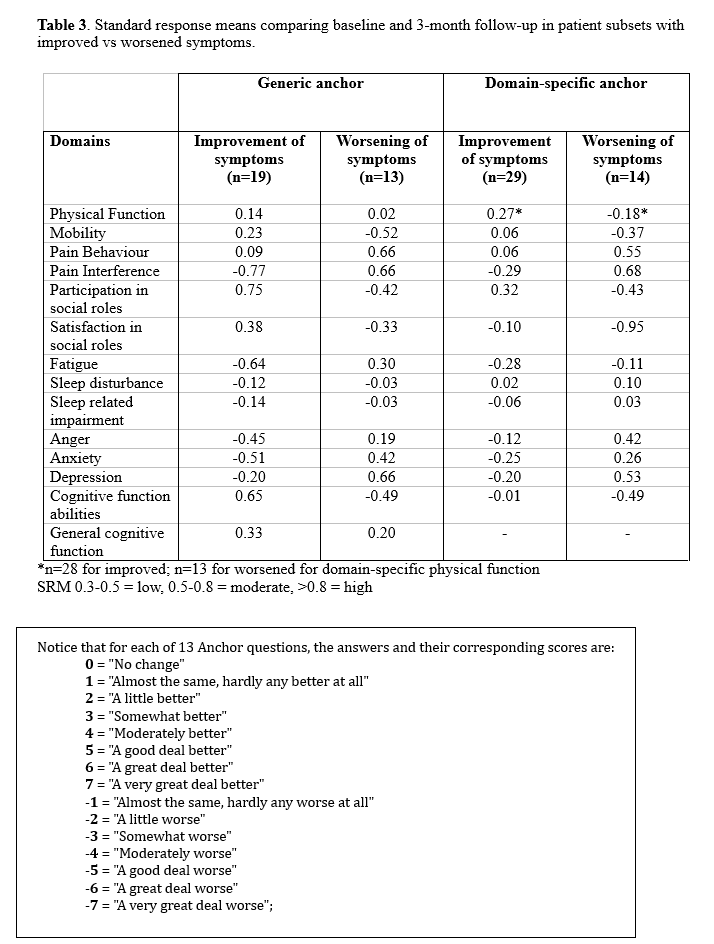
With respect to the general anchor, of 14 PROMIS domains, 8 demonstrated moderate SRMs: mobility, pain behaviour, pain interference, participation in social roles, fatigue, anxiety, depression, and cognitive function abilities (Table 3).
For domain-specific anchors (Table 3), only 3 domains (pain behaviour, pain interference, depression) demonstrated moderate SRMs. Only 1 domain (satisfaction in social roles) had a high SRM. SRM were overall larger with the generic anchor compared to domain-specific anchor.
Conclusion: Out of 14 PROMIS domains, 8 were sensitive to change at 3-month follow-up when compared to a generic anchor question. In contrast, for domain-specific anchors, only 4 domains were sensitive to change at 3-month follow-up, of which one had not been sensitive in the generic anchor analysis. This highlights the importance of using appropriate anchor questions when assessing responsiveness. Focusing on patients with more significant reported changes (+/- 4-7 on anchors) may yield larger SRMS. This requires a larger sample size and currently cannot be performed. Further work is needed to evaluate the utility of PROMIS CAT measures in the care of lupus patients as well as to determine minimal clinically important differences.
To cite this abstract in AMA style:
Fung W, Moazzami M, Engel L, Su J, Bonilla D, Akhavan P, Katz P, Beaton D, Touma Z. Sensitivity to Change of the Patient Reported Outcomes Measurement Information System (PROMIS) Computerized Adaptive Test (CAT) Measures in a Single Canadian Lupus Cohort [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/sensitivity-to-change-of-the-patient-reported-outcomes-measurement-information-system-promis-computerized-adaptive-test-cat-measures-in-a-single-canadian-lupus-cohort/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/sensitivity-to-change-of-the-patient-reported-outcomes-measurement-information-system-promis-computerized-adaptive-test-cat-measures-in-a-single-canadian-lupus-cohort/