Session Information
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: The calcineurin inhibitors (CNI) cyclosporine (CSA) and tacrolimus (TAC) were revolutionary when first introduced for solid organ transplant. Voclosporin (VCS), a novel CNI, is the first oral therapy approved for the treatment of active lupus nephritis. Unlike CSA and TAC, VCS has demonstrated consistent pharmacokinetics and pharmacodynamics, eliminating the need for therapeutic drug monitoring. Further, VCS is associated with a more favorable metabolic profile and has not been associated with electrolyte disturbances.
Emerging evidence indicates small molecule therapies display differential disposition within organ tissues. This suggests that CNIs may be differentially distributed and retained in the kidney, potentially explaining differences in their efficacy and safety. Here we assessed in mice and humans the disposition of each CNI in the kidney relative to its systemic drug exposure.
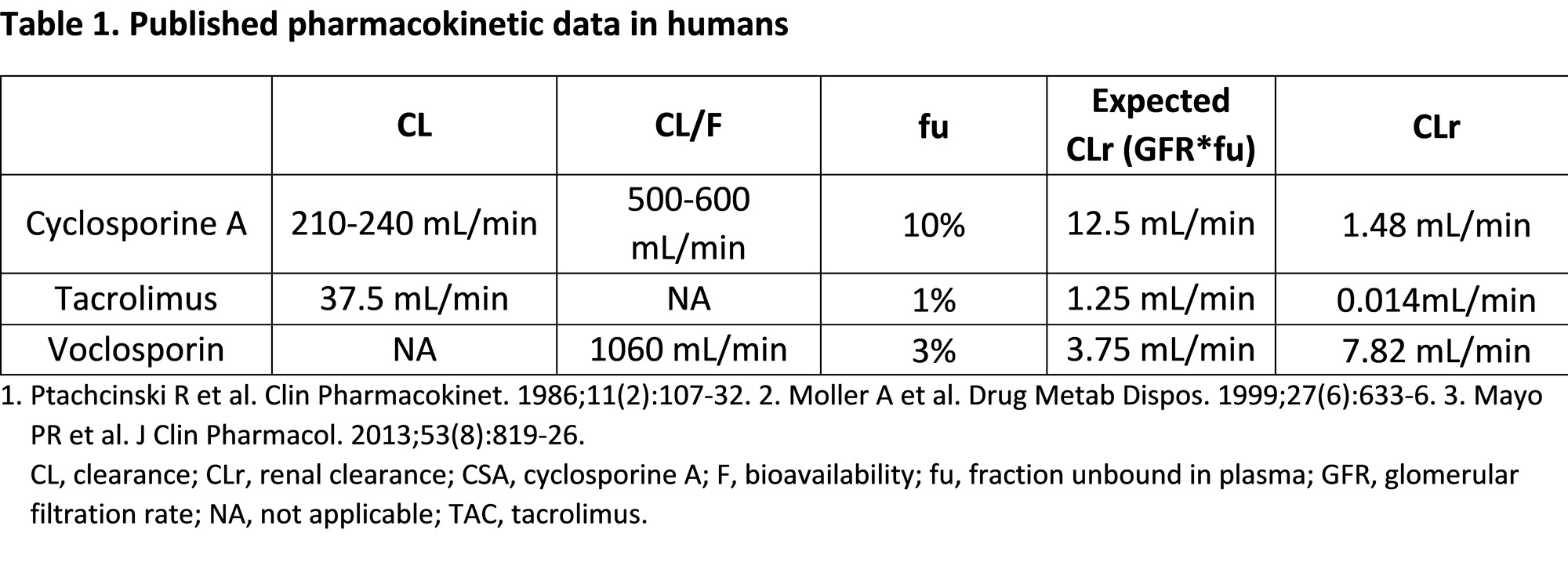
Methods: Single 30 mg/kg doses of CSA, TAC and VCS were administered intravenously (IV) to mice. Following IV administration, kidneys were collected at various time points, flash frozen in liquid nitrogen, and stored at -20 0C. Sections of kidney tissue were mounted on indium tin oxide coated glass slides. Matrix of 10 mg/mL α-Cyano-4-hydroxycinnamic acid in 85% acetonitrile/13% ethanol + 2% water + 0.1% trifluoroacetic acid was sprayed on the tissue, dried for 10 minutes, and subjected to Matrix-assisted Laser Desorption and Ionization Mass Spectrometry Imaging (MALDI-MSI). The systemic and renal clearance (CLr) in humans of CSA and TAC were obtained from literature; pharmacokinetic data on VCS was obtained from data on file. Renal secretion of each drug was compared to its expected passive filtration based on glomerular filtration rate (GFR), fraction unbound in plasma (fu), and respective systemic drug exposure.
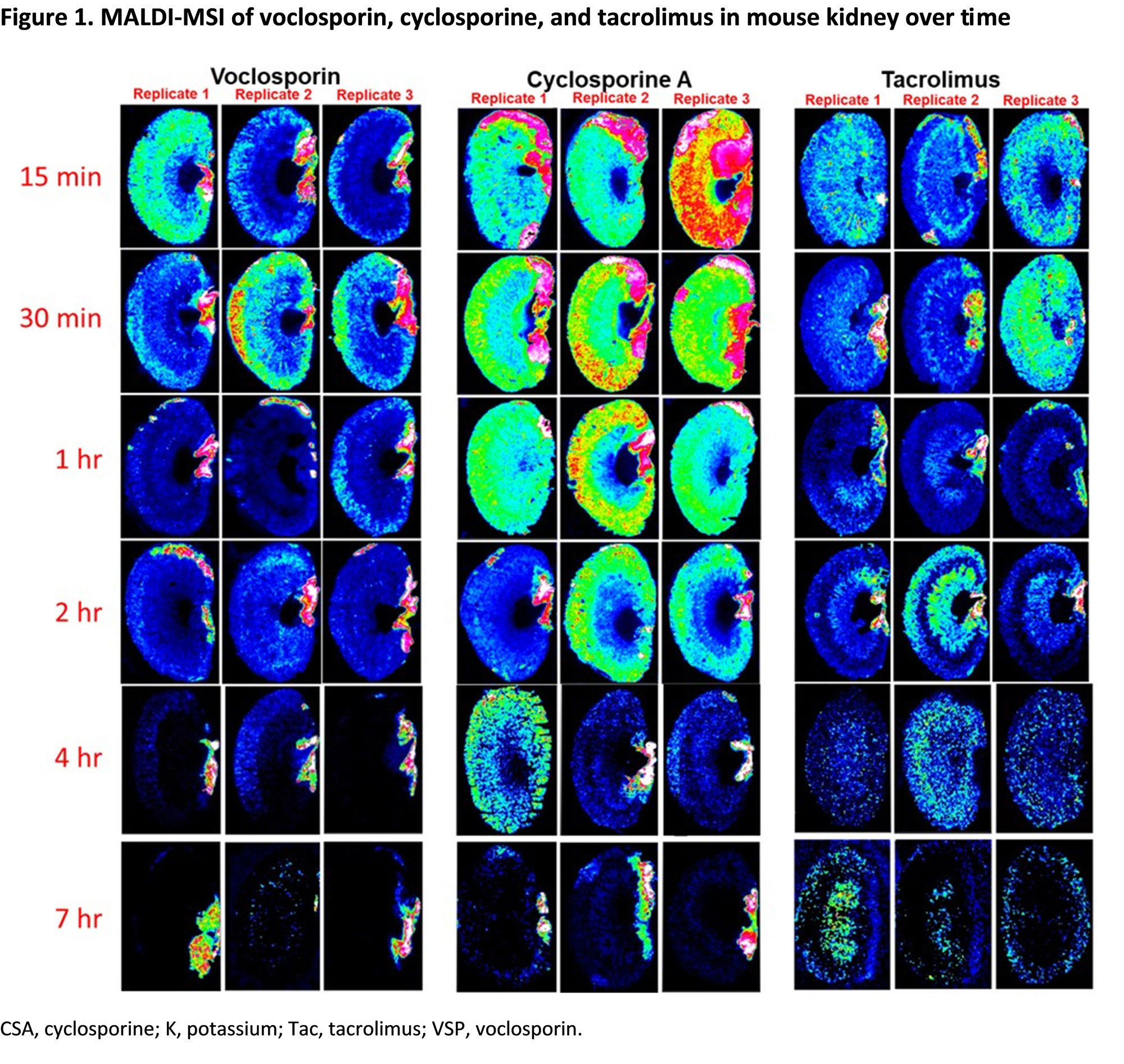
Results: MALDI-MSI demonstrated significantly higher concentrations of drug and more diffuse tissue disposition of CSA in mouse kidney compared to VCS (Figure 1). CSA was retained up to 2 hrs post-administration. Higher concentrations and more diffuse disposition of TAC was also noted compared to VCS at 15 and 30 min; TAC was distinctively retained in the cortex and medulla. VCS had moderate distribution in the cortex and was rapidly excreted with low levels present in the kidney after 1 hr.
According to published data, CSA has a measured renal CLr of 1.48 mL/min in humans, representing approximately 10% of expected passive filtration of 12.5 mL/min (Table 1). TAC has a CLr of 0.014 mL/min representing < 2% of expected passive filtration of 1.25 mL/min. VCS has a CLr of 7.82 mL/min representing approximately 200% of its expected passive filtration rate of 3.75 mL/min.
Conclusion: MALDI-MSI revealed differential retention and distribution of CSA, TAC and VCS in mice, consistent with their CLr in humans. Higher drug exposure and >90% renal reabsorption was observed for CSA and TAC, whereas renal handling of VCS suggested significant tubular secretion. The higher rate of secretion and lower overall renal exposure to VCS may be associated with improved safety when compared to the more diffuse distribution and greater renal retention of CSA and TAC.
To cite this abstract in AMA style:
Zhou S, Kumari Rajanayake K, He M, Wen B, Lkhagva A, Yap E, Sun D, Cross J, Engelke K, Huizinga R. Selective Disposition of Voclosporin, Cyclosporine, and Tacrolimus in Renal Tissue [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/selective-disposition-of-voclosporin-cyclosporine-and-tacrolimus-in-renal-tissue/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/selective-disposition-of-voclosporin-cyclosporine-and-tacrolimus-in-renal-tissue/