Session Information
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Real-world data on long-term use of secukinumab complements clinical trial findings by providing insights from diverse patients in routine clinical settings. SERENA (CAIN457A3403) was a non-interventional, prospective study conducted across 19 primarily European countries for up to 5 years in patients with moderate to severe chronic plaque psoriasis, active psoriatic arthritis (PsA) or radiographic axial spondyloarthritis (r-axSpA), who had received secukinumab for ≥16 weeks before enrolment. Here, we report the final 5-year results of retention and effectiveness of secukinumab in patients with active PsA or r-axSpA from the study.
Methods: Secukinumab retention rate was derived from Kaplan-Meier estimates for the proportion of patients who had been treated with secukinumab at years 1, 2, 3, 4 and 5. Effectiveness assessments included swollen joint count (SJC) and tender joint count (TJC) in patients with PsA, Patient Global Assessment (PtGA) of disease activity of Numeric Rating Scale (NRS ≤2) and Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) score in patients with r-axSpA, up to 5 years.
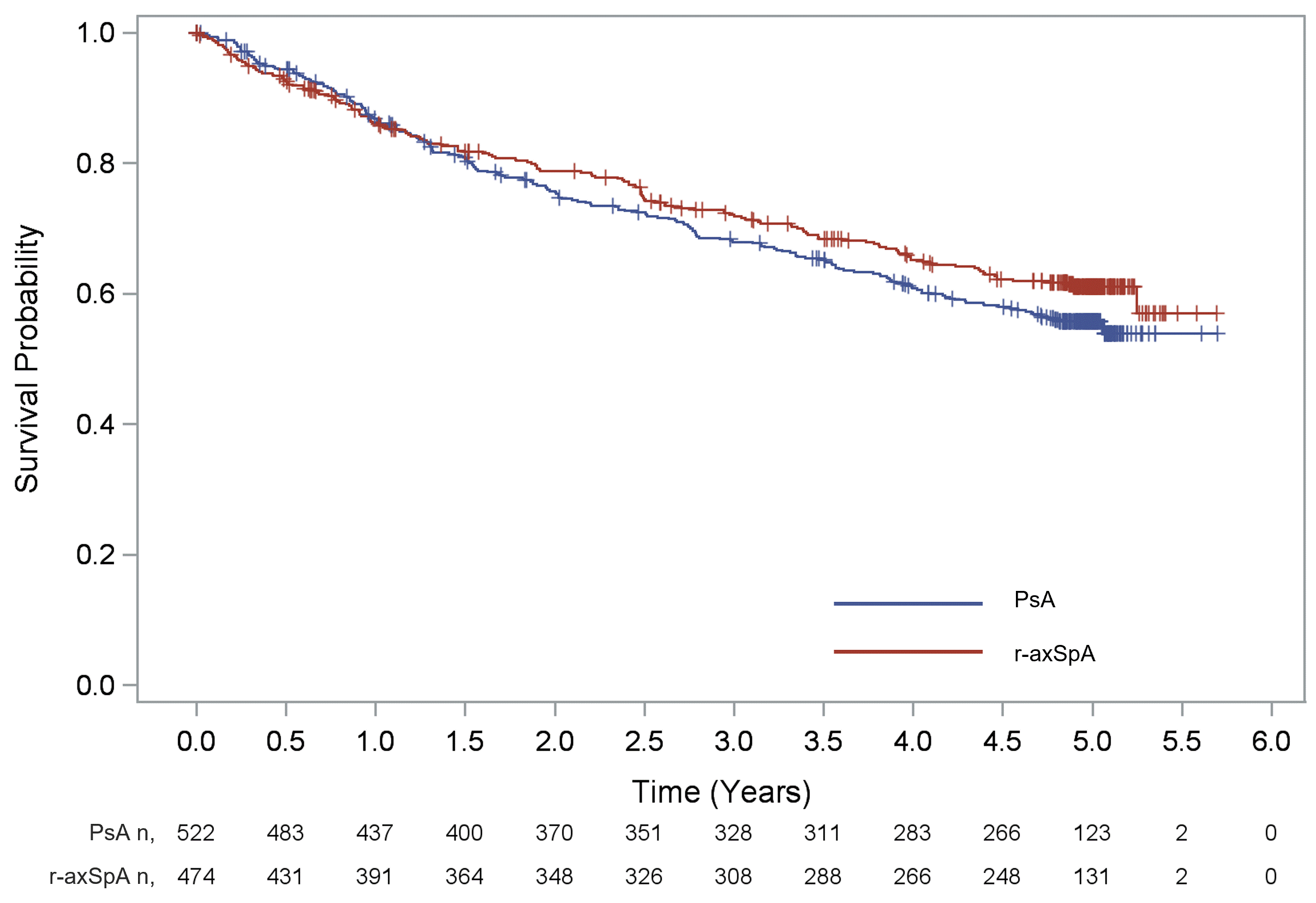
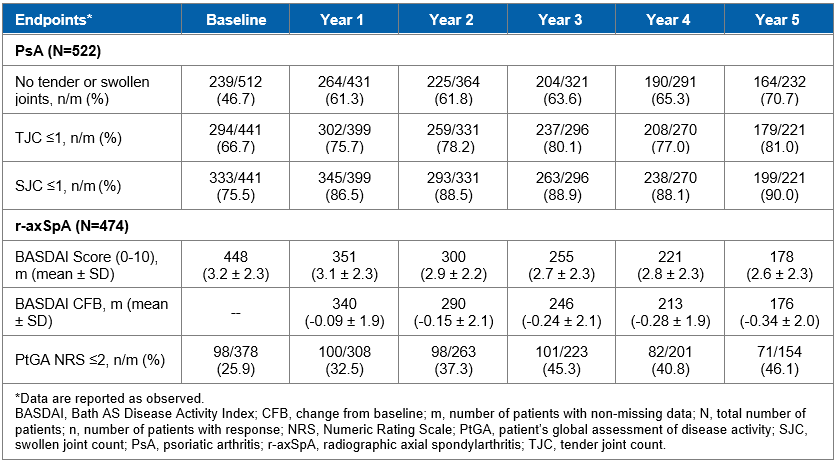
Results: Overall, 522 patients with PsA and 474 patients with r-axSpA were included in the analysis. The mean age at inclusion was 52.5 years in the PsA group and 46.5 years in the r-axSpA group, with 44.8% and 60.5% being male, respectively. Mean body mass index of 28.7 kg/m2 was observed in the PsA group and 27.0 kg/m2 in the r-axSpA group. Disease duration at enrolment was 8.6 ± 7.4 years in PsA and 9.8 ± 9.5 years in r-axSpA respectively. Overall, 68.6% and 64.6% of patients with PsA and r-axSpA had undergone bDMARDs (biologic disease-modifying antirheumatic drugs) treatment prior to secukinumab, respectively. Before inclusion in the study, the patients had been receiving secukinumab treatment for an average of 1 year. After inclusion in the study, the treatment retention rates at years 1, 2, 3, 4 and 5 for PsA group were 86.9%, 75.8%, 67.9%, 60.8% and 55.7%, respectively (Figure 1). Similarly, the treatment retention rates at years 1, 2, 3, 4 and 5 for r-axSpA group were 86.2%, 78.8%, 72.1%, 65.2% and 61.1%, respectively (Figure 1). The most common reasons for discontinuation in PsA and r-axSpA were lack of efficacy (27.2% and 17.7%, respectively), patient decision (11.9% and 8.6%), lost to follow-up (5.7% and 6.1%) and adverse events (3.1% and 7.2%). The proportion of patients with TJC ≤1 was 75.7% at year 1 and 81.0% at year 5; the proportion of patients with a SJC ≤1 response was 86.5% at year 1 and 90.0% at year 5 in the PsA group (Table 1). BASDAI improved in r-axSpA group through the 5 years. The proportion of patients with PtGA NRS ≤2 was 32.5% at year 1 and 46.1% at year 5 in the r-axSpA group (Table 1).
Conclusion: Secukinumab retention rates were high with sustained effectiveness in patients with PsA and r-axSpA during 5-year follow-up in a prospective real-world setting.
n, number of patients at risk, PsA, psoriatic arthritis, r-axSpA, radiographic axial spondylarthritis.
To cite this abstract in AMA style:
Kiltz U, Sfikakis P, Bounas A, Gullick N, LESPESSAILLES E, Brandt Jürgens J, Rashkov R, Schulz B, Bao W, Jagiello P, Gaffney K. Secukinumab Retention and Effectiveness in Patients with Psoriatic Arthritis and Radiographic Axial Spondyloarthritis: 5-year Final Results of a Prospective Real-world Study [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/secukinumab-retention-and-effectiveness-in-patients-with-psoriatic-arthritis-and-radiographic-axial-spondyloarthritis-5-year-final-results-of-a-prospective-real-world-study/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/secukinumab-retention-and-effectiveness-in-patients-with-psoriatic-arthritis-and-radiographic-axial-spondyloarthritis-5-year-final-results-of-a-prospective-real-world-study/