Session Information
Date: Monday, October 27, 2025
Title: (1147–1190) Miscellaneous Rheumatic & Inflammatory Diseases Poster II
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: GCA and PMR are closely related, immune-mediated chronic inflammatory diseases often occurring concurrently in individuals over 50.1,2 While glucocorticoids (GC) are the mainstay of treatment, relapses are common, and long-term use causes significant side effects, highlighting the need for GC-sparing therapies.2 Secukinumab (SEC), a monoclonal antibody that selectively neutralizes interleukin-17A, has shown efficacy and safety in various immune-mediated diseases.3 In the TitAIN phase 2 study, SEC resulted in a higher sustained remission rate in patients with GCA than in placebo (PBO) at week 28, with effects lasting for 52 weeks; safety was consistent with its known safety profile.4 This post hoc analysis evaluated SEC vs PBO in the subgroup of GCA patients with PMR symptoms within 12 weeks prior to and/or at baseline (BL).
Methods: TitAIN was a randomized, double-blind, PBO-controlled, multicenter, phase 2 study in patients aged ≥50 years with new-onset or relapsing GCA (unequivocal cranial symptoms of GCA and/or symptoms of PMR) who were naive to biological therapy and receiving GCs at BL with a prednisolone equivalent dose of 25–60 mg/day.4 Patients were assigned (1:1) to receive 300 mg SEC or PBO subcutaneously once a week up to week 4 and every 4 weeks thereafter. In both arms, prednisolone dose was tapered to 0 mg over a 26-week period.4
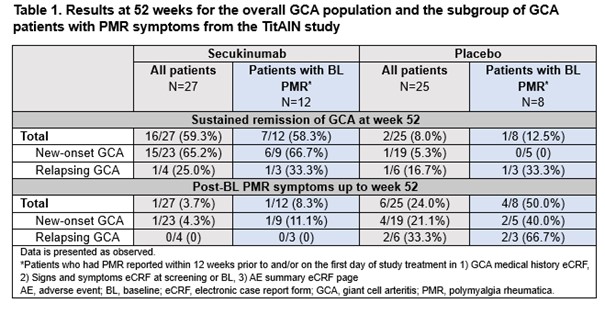
Results: In the TitAIN study, 27 patients received SEC and 25 received PBO; 44.4% (12/27) of patients in the SEC arm vs 32.0% (8/25) in the PBO arm had PMR symptoms and were included in this post hoc analysis. Among patients with PMR symptoms, 58.3% (7/12) in the SEC arm vs 12.5% (1/8) in the PBO arm were in sustained GCA remission at week 52, consistent with a previous finding in the overall study population (59% vs 8%).4Overall, only 3.7% (1 of 27) in the SEC arm vs 24.0% (6/25) in the PBO arm experienced PMR symptoms post BL up to week 52. In the subgroup of patients with PMR symptoms, only 8.3% (1/12) in the SEC arm had PMR symptoms post BL compared to 50.0% (4/8) in the PBO arm. Data for new-onset and relapsing GCA subgroups are presented in Table 1.All GCA patients with PMR symptoms had ≥1 adverse event (AE), in both arms. The most common AEs were consistent with those reported in the study population.4 Serious AEs were reported in 8.3% of patients (n=1; facial paralysis) in the SEC arm and 37.5% (n=3; gastrointestinal pain, dizziness, and chronic obstructive pulmonary disease) in the PBO arm.
Conclusion: This post hoc analysis showed a numerical reduction in patients with PMR symptoms with SEC treatment compared to PBO. The safety profile of SEC was similar to the overall GCA study population and consistent with its known safety profile. These results support the development of SEC for PMR. A phase 3 trial (NCT05767034) is evaluating the efficacy and safety of SEC in relapsed patients with PMR.ReferencesEspígol-Frigolé G, et al. Lancet. 2023;402(10411):1459-72.Man-Ger Sun M, et al. Best Pract Res Clin Rheumatol. 2022;36(4):101822.COSENTYX®. Prescribing information. Novartis; 2023. Accessed on April 29, 2025. Available at: label.Venhoff N, et al. Lancet Rheumatol 2023;5:e341-50.
To cite this abstract in AMA style:
Venhoff N, Schmidt W, Bergner R, Rech J, Unger L, Finzel S, Andreica I, Kofler D, Weiner S, Lamprecht P, Schulze-Koops H, Mendelson M, Bao W, Keyport M, Maricos M, Jordan M. V, Thiel J. Secukinumab In Patients with Giant Cell Arteritis with Polymyalgia Rheumatica Symptoms: A Post Hoc Analysis of the Phase 2 TitAIN Study [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/secukinumab-in-patients-with-giant-cell-arteritis-with-polymyalgia-rheumatica-symptoms-a-post-hoc-analysis-of-the-phase-2-titain-study/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/secukinumab-in-patients-with-giant-cell-arteritis-with-polymyalgia-rheumatica-symptoms-a-post-hoc-analysis-of-the-phase-2-titain-study/