Session Information
Date: Monday, November 6, 2017
Title: Spondyloarthropathies and Psoriatic Arthritis – Clinical Aspects and Treatment Poster II
Session Type: ACR Poster Session B
Session Time: 9:00AM-11:00AM
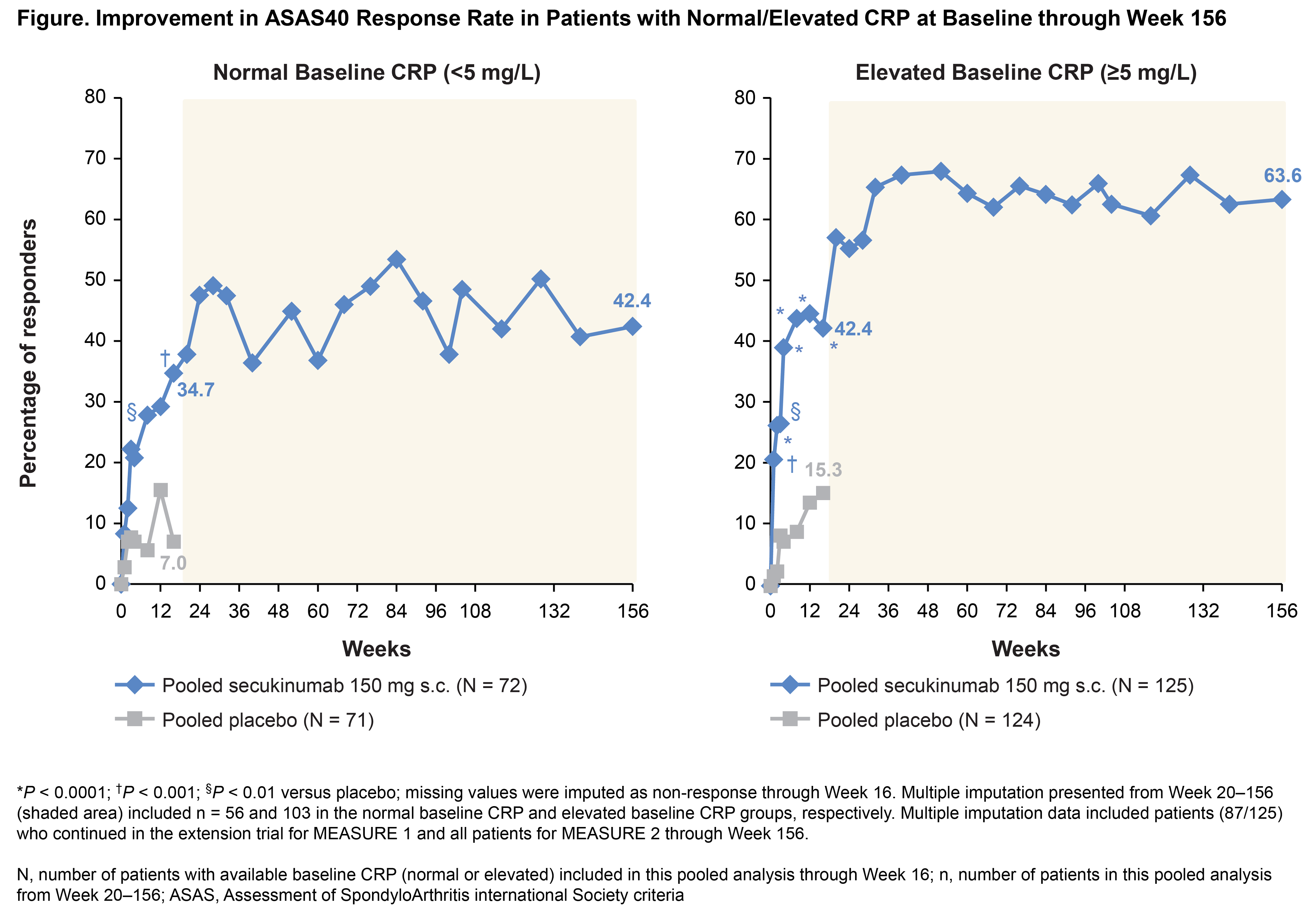
Background/Purpose: Inhibition of IL-17A with secukinumab is an approved therapy for patients (pts) with AS.1 It has been previously reported that response rates with TNFα inhibitors (TNFi) are higher in AS pts with elevated baseline (BL) CRP levels;2-4 however, this relationship is unclear for IL‒17A-inhibition. This post-hoc analysis assessed the response to secukinumab treatment in AS pts with normal or elevated BL CRP from the phase 3 MEASURE 1 and MEASURE 2 studies over 3 years.
Methods: The study designs of MEASURE 1 and 2 have been reported elsewhere.1 This analysis pooled data from all pts with available BL CRP levels in the 2 studies who received subcutaneous (s.c.) secukinumab 150 mg (approved dose; N = 197) or placebo (PBO; N = 195). Efficacy endpoints included ASAS20/40, BASDAI, BASDAI50, AS disease activity score (ASDAS) inactive disease, and ASAS partial remission (ASAS PR) in pts stratified by BL CRP as defined by the central lab: normal (<5 mg/L) or elevated (≥5 mg/L). Data are presented using non-responder imputation through Week (Wk) 16 and multiple imputation from Wk 20‒156 for binary variables, and mixed-effect model repeated measure for all time points through Wk 156 for continuous variables.
Results: Overall, 36.5% (143/392) of pts with normal CRP and 63.5% (249/392) of pts with elevated CRP at BL were included in this pooled analysis. The proportion (36% and 64%) of pts with normal and elevated BL CRP was similar in the secukinumab 150 mg and PBO groups. BL demographic/clinical characteristics were balanced across the normal and elevated BL CRP groups (mean age ± SD: 43.8 ± 12.0 and 41.0 ± 12.5 years, mean time since diagnosis ± SD: 7.0 ± 8.7 and 7.2 ± 7.9 years, mean BASDAI ± SD: 6.7 ± 1.5 and 6.4 ± 1.5, respectively). At Wk 16, the ASAS20 and ASAS40 response rates were improved with secukinumab 150 mg vs PBO in pts with normal or elevated BL CRP (Figure and Table). The treatment effect of secukinumab 150 mg vs PBO at Wk 16 was significant in pts with a normal or elevated BL CRP for all other outcomes, except for ASAS PR in the normal BL CRP group (P = 0.07; Table). Results were sustained or further improved through 156 wks of secukinumab treatment in both groups.
Conclusion: Secukinumab 150 mg demonstrated efficacy in AS pts with either normal or elevated BL CRP, with greater magnitude of response in pts with elevated BL CRP.
References: 1. Baeten D, et al. N Engl J Med. 2015;373:2534‒48; 2. Rudwaleit M, et al. Ann Rheum Dis. 2008;67:1276–81; 3. Landewé R, et al. Ann Rheum Dis. 2014;73:39–47; 4. Rudwaleit M, et al. J Rheumatol. 2009;36;801–8.
|
Table. Summary of Clinical Efficacy |
|||||
|
|
|
Normal Baseline CRP (<5 mg/L) |
Elevated Baseline CRP (≥5 mg/L) |
||
|
Clinical outcomes |
Week |
Pooled secukinumab 150 mg s.c. (N = 72) |
Pooled PBO (N = 71) |
Pooled secukinumab 150 mg s.c. (N = 125) |
Pooled PBO (N = 124) |
|
ASAS20, % |
16 |
56.9§ |
28.2 |
63.2* |
29.0 |
|
156 |
61.3 |
– |
78.3 |
– |
|
|
ASAS40, % |
16 |
34.7† |
7.0 |
42.4* |
15.3 |
|
156 |
42.4 |
– |
63.6 |
– |
|
|
BASDAI, mean change from baseline |
16 |
−2.2† |
-1.0 |
−2.4* |
−0.6 |
|
156 |
−2.7 |
– |
−3.4 |
– |
|
|
BASDAI50, % |
16 |
27.8§ |
7.0 |
39.2* |
10.5 |
|
156 |
40.4 |
– |
60.6 |
– |
|
|
ASDAS |
16 |
19.4‡ |
4.2 |
15.2§ |
2.4 |
|
156 |
28.9 |
– |
19.6 |
– |
|
|
ASAS PR, % |
16 |
12.5 |
2.8 |
16.0§ |
4.0 |
|
156 |
14.4 |
– |
32.1 |
– |
|
|
*P < 0.0001; †P < 0.001; §P < 0.01; ‡P < 0.05 versus placebo; missing values were imputed as non-response at Week 16. Multiple imputation and MMRM presented at Week 156 included n = 56 and 103 in the normal baseline CRP and elevated baseline CRP groups, respectively. Multiple imputation and MMRM data included patients (87/125) who continued in the extension trial for MEASURE 1 and all patients for MEASURE 2 at Week 156. For BASDAI, LS mean change from baseline was presented using MMRM at Weeks 16 and 156. ASAS, Assessment of SpondyloArthritis international Society criteria; ASDAS, Ankylosing Spondylitis Disease Activity Score; BASDAI, Bath Ankylosing Spondylitis Disease Activity Index; LS, least squares; MMRM, mixed-effect model repeated measures; N, number of patients with available baseline CRP (normal or elevated) included in this pooled analysis at Week 16; n, number of patients in this pooled analysis at Week 156; PBO, placebo; PR, partial remission |
|||||
To cite this abstract in AMA style:
Braun J, Sieper J, Landewé RBM, Baraliakos X, Miceli-Richard C, Martin R, Porter B, Gandhi K, van der Heijde D. Secukinumab Demonstrates Rapid and Sustained Efficacy in Ankylosing Spondylitis Patients with Normal or Elevated Baseline CRP Levels: Pooled Analysis of Two Phase 3 Studies [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 10). https://acrabstracts.org/abstract/secukinumab-demonstrates-rapid-and-sustained-efficacy-in-ankylosing-spondylitis-patients-with-normal-or-elevated-baseline-crp-levels-pooled-analysis-of-two-phase-3-studies/. Accessed .« Back to 2017 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/secukinumab-demonstrates-rapid-and-sustained-efficacy-in-ankylosing-spondylitis-patients-with-normal-or-elevated-baseline-crp-levels-pooled-analysis-of-two-phase-3-studies/