Session Information
Session Type: Late-Breaking Abstract Session
Session Time: 4:00PM-6:00PM
Background/Purpose: Non-radiographic axial spondyloarthritis (nr-axSpA) and ankylosing spondylitis (AS) are considered part of the spectrum of axSpA. Patients (pts) are classified as nr-axSpA due to absence of definitive radiographic sacroiliitis but suffer from similar disease burden as pts with AS. NSAIDs are first-line therapy, and one anti-TNF therapy is approved for nr-axSpA pts with objective signs of inflammation in the US1. Secukinumab (SEC) provides significant and sustained improvement in signs and symptoms of pts with AS2.
Methods: PREVENT (NCT02696031) is the first phase 3 study evaluating the efficacy and safety of SEC 150 mg with (LD) or without loading (NL) in pts with nr-axSpA. The study had 2 independent analysis plans as per EU (Week [Wk] 16) and US (Wk 52) regulatory requirements. Here, the Wk 16 efficacy results and safety to Wk 20 from the EU analysis plan are reported. This phase 3, double blind, placebo (PBO)-controlled, multicenter trial included 555 pts (aged ≥18 years) fulfilling the ASAS classification criteria for axSpA plus abnormal CRP and/or MRI, with no radiographic changes in the sacroiliac joints meeting modified New York Criteria. All images were assessed by central reading. Pts were randomized (1:1:1) to subcutaneous SEC 150 mg LD, 150 mg NL, or PBO. The LD group received SEC 150 mg at baseline (BL), Wks 1, 2, 3, and 4 , and then every 4 wks (q4wk) through Wk 52. NL group received SEC 150 mg at BL and PBO at Wks 1, 2, and 3, and then SEC 150 mg q4wk starting at Wk 4. PBO group received PBO at BL, Wks 1, 2, 3 and 4 and then q4wk. The primary endpoint was ASAS40 response with SEC 150 mg LD in anti-TNF-naïve pts at Wk 16. Secondary endpoints included ASAS40 response rates, total BASDAI, BASDAI50, BASFI, SF-36 PCS, ASQoL, and ASAS partial remission in the overall population. Analysis used NRI for binary and MMRM for continuous variables. Endpoints were analyzed according to a statistical hierarchy. Safety analyses included all pts who received ≥1 dose of study treatment.
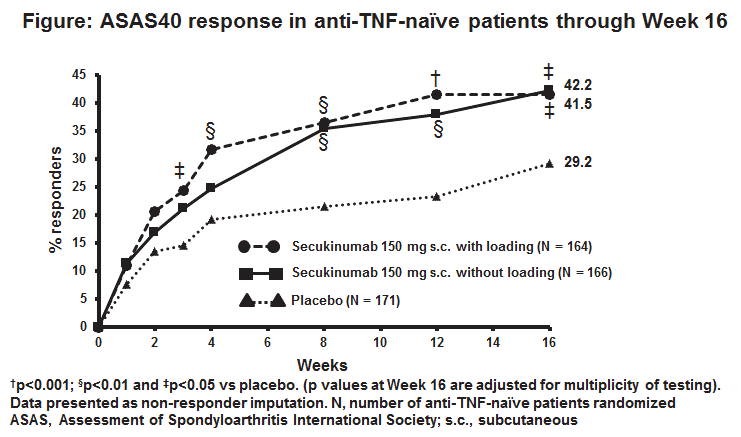
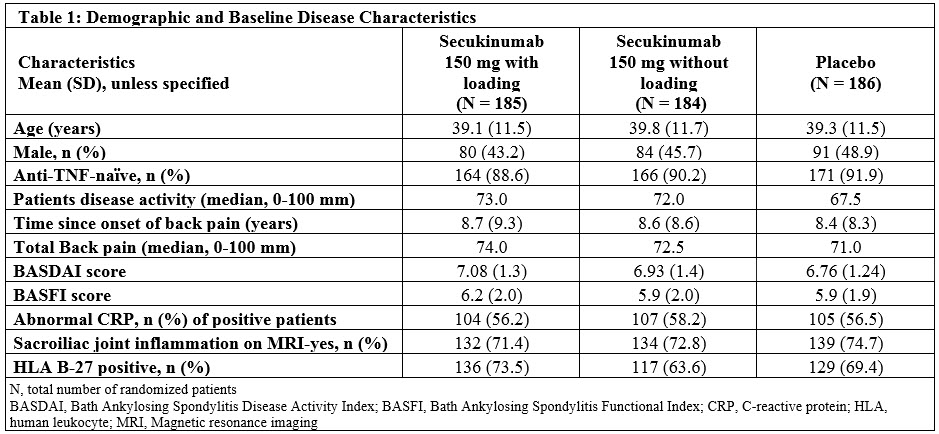
Results: 94.6% (175/185; 150 mg LD), 96.2% (177/184; 150 mg NL) and 94.1% (175/186; PBO) pts completed 24 wks of treatment. Demographic and baseline disease characteristics were comparable across groups (Table 1). The primary and all secondary endpoints were met. At Wk 16, ASAS40 in anti-TNF-naïve pts was significantly higher in the SEC 150 mg LD group than PBO (Figure). SEC 150 mg LD and NL also showed significant improvement vs PBO in all secondary endpoints (Table 2). Three cases of serious infections/infestations (0.8%), and 1 case of Crohn’s disease (0.3%) were reported with SEC. No cases of oesophageal candidiasis, major adverse cardiovascular events, malignancy or death were reported on any SEC dose up to Wk 20.
Conclusion: PREVENT is the first randomized controlled trial evaluating the efficacy and safety of SEC in pts with nr-axSpA. SEC 150 mg LD and NL provided significant improvement in signs and symptoms of nr-axSpA through Wk 16. The safety profile of SEC was consistent with the established safety profile across indications3.
References:
- van der Heijde D, et al. Ann Rheu Dis. 2017;76:978-991.
- Lubrano E and Perrotta FM. Ther Clin Risk Manag. 2016;12:1587-92.
- Deodhar A, et al. Arth Res Ther. 2019;21:111.
To cite this abstract in AMA style:
Deodhar A, Blanco R, Dokoupilova E, van de Sande M, Hall S, Wiksten A, Porter B, Richards H, Haemmerle S, Braun J. Secukinumab 150 mg Significantly Improved Signs and Symptoms of Non-radiographic Axial Spondyloarthritis: Results from a Phase 3 Double-blind, Randomized, Placebo-controlled Study [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/secukinumab-150-mg-significantly-improved-signs-and-symptoms-of-non-radiographic-axial-spondyloarthritis-results-from-a-phase-3-double-blind-randomized-placebo-controlled-study/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/secukinumab-150-mg-significantly-improved-signs-and-symptoms-of-non-radiographic-axial-spondyloarthritis-results-from-a-phase-3-double-blind-randomized-placebo-controlled-study/

.jpg)