Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: The Outcome Measures in Rheumatology (OMERACT) Systemic Lupus Erythematosus (SLE) Working Group was re-established in 2018 to update the SLE Core Outcome Set (COS) and includes over 260 members representing 6 continents and over 30 countries. A COS is a fundamental list of outcomes to standardize the measurement and reporting of domains/outcomes in SLE trials. The first SLE COS was developed in 1998, thus there is a need to update the SLE COS. Three years ago, the OMERACT SLE Working Group embarked on multiple projects to generate an extensive list of potential candidate domains to be included in the SLE COS. In this study, we specifically report on the scoping literature review results.
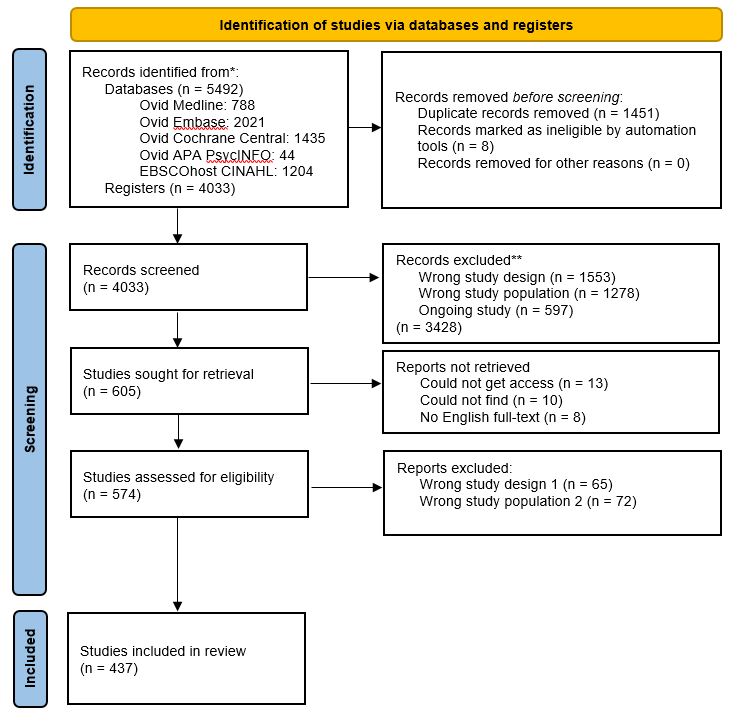
Methods: Scoping Review: We searched 5 databases (Medline, Embase, Cochrane, CINALH, PsycINFO) for SLE systematic reviews and clinical trials since 2010. 3 reviewers performed an agreement test on 100 articles for title and abstract screening using Covidence with a 98% agreement. Due to the large volume of studies, the 1st screening of title and abstract was conducted by a single reviewer. A 2nd agreement test on 100 articles was performed for full-text screening with the same reviewers with a 99% agreement. The 2nd screening of full-texts and data extraction of domain items consisted of 2 reviewers per article.
Advisory Group: An advisory group was established with OMERACT SLE Working Group Steering Committee members, Patient Research Partners, and additional SLE Expert Collaborators. This group reviewed all domain items generated from the scoping literature review, winnowing any domain items deemed too broad, narrow, multivariable, non-specific, or contextual factors, and binning the remaining domain items into preliminary candidate domains.
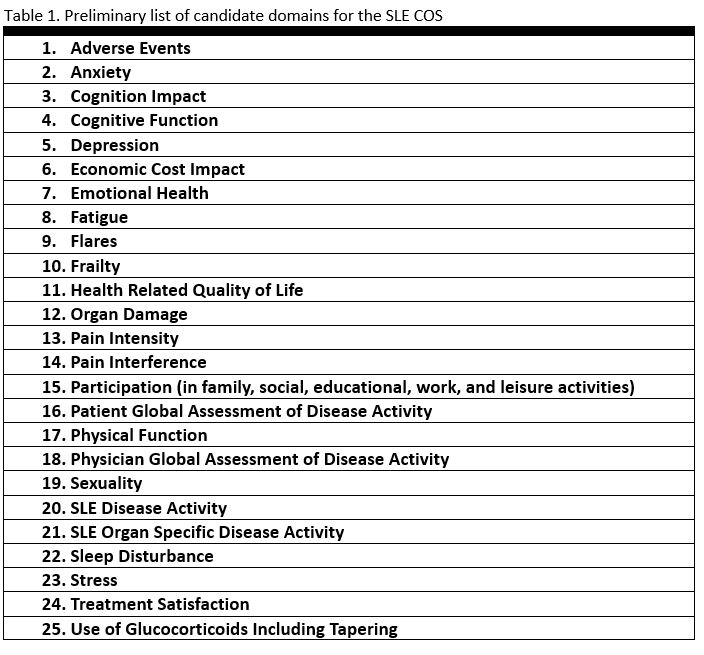
Results: The scoping literature review search yielded 4033 articles which title and abstract screening reduced to 605 (Figure 1). Article retrieval and full-text review further reduced the number of articles to 437. All domain items were extracted and winnowed and binned along with the results of other domain generation projects into 25 preliminary candidate domains. Related themes/domains were grouped and contextual factors were removed yielding a preliminary list of 25 domains (Table 1).
Conclusion: This scoping review identified multiple domain items expanding the list of preliminary candidate domains to 25. Future steps for SLE COS development include further generation of potential domains from qualitative research, to be followed by definition development for candidate domains in preparation for the final step in domain selection – the Delphi consensus exercise to vote on the final SLE COS.
To cite this abstract in AMA style:
Nielsen W, Kharouf F, Munoz-Grajales C, Thayaparan A, Anderson M, Simon L, Desai M, Parodis I, Kim A, Enman Y, Bingham K, Bonilla D, Thumboo J, Mosca M, Aringer M, Morand E, Bruce I, Strand V, Touma Z. Scoping Literature Review to Identify Candidate Domains for the SLE OMERACT Core Outcome Set [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/scoping-literature-review-to-identify-candidate-domains-for-the-sle-omeract-core-outcome-set/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/scoping-literature-review-to-identify-candidate-domains-for-the-sle-omeract-core-outcome-set/