Session Information
Date: Tuesday, November 14, 2023
Title: (2257–2325) SLE – Diagnosis, Manifestations, & Outcomes Poster III
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: We established the OMERACT SLE Working Group in 2021 which includes over 150 members representing over 25 countries and 5 continents to develop a new SLE Core Domain Set (CDS) and measurement instrument set. A CDS is essential for standardizing the measurement and reporting of domains in clinical trials and longitudinal studies in SLE. Domains in a CDS are selected from a candidate domain list through consensus voting exercises with stakeholders including patients, clinicians, researchers, pharmaceutical representatives, and others. The first CDS development stage is generating a preliminary list of candidate domains. To inform the development of the new OMERACT SLE CDS, we have conducted a scoping literature review of systematic reviews and clinical trials of SLE, and focus groups with SLE patients about living with SLE.
Methods: Scoping Review: We searched 5 databases (Medline, Embase, Cochrane, CINALH, PsycINFO) since 2010 and used Covidence for article screening. 3 reviewers performed an agreement test on the first 100 articles for title and abstract screening with a 98% agreement. Due to the large volume of studies, the 1st screening of title and abstract was conducted by a single reviewer. A 2nd agreement test on 100 articles was performed for full-text screening with the same reviewers with a 99% agreement. The 2nd screening of full-texts and data extraction of domains required 2 reviewers per article. Focus Groups: We developed an interview guide of 11 open-ended questions asking about the pathophysiological manifestations and life impact of SLE. Patients were recruited by the University of Toronto from around the world representing 5 continents and 13 countries. Six focus groups were held virtually ranging from 3 to 10 participants per group. Transcripts have been developed and thematically coded using an open coding framework. Advisory Group: An advisory group was established with members from the OMERACT SLE Working Group Steering Committee, Patient Research Partners, and additional SLE Expert Stakeholders. This group reviewed all domains and themes and sorted them into domains.
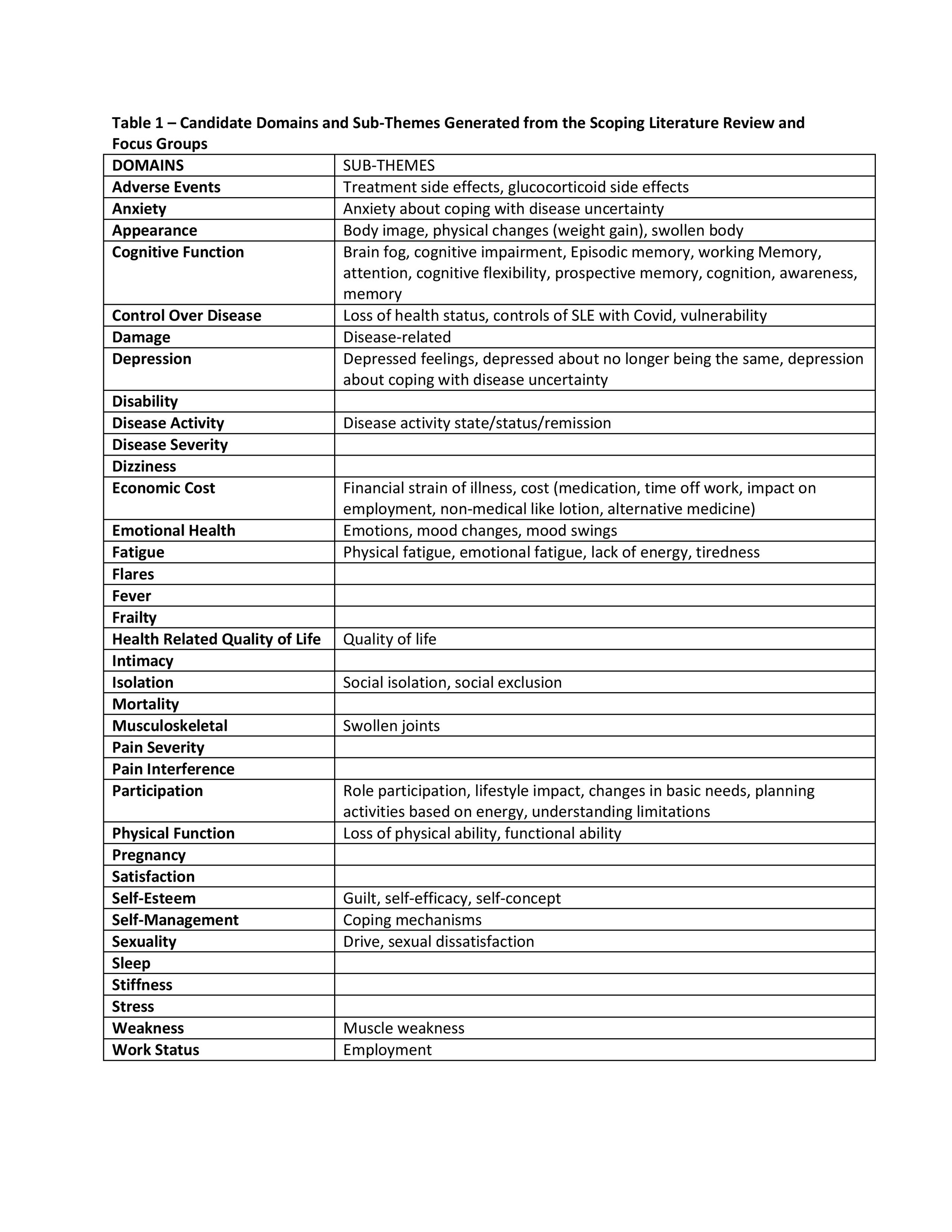
Results: The literature search yielded 4113 articles. The 1st screening reduced the number of articles to 1421. The 2nd screening reduced the number of articles to 597. The scoping review and focus group transcripts yielded over 200 themes/domains. Duplicate and related themes/domains were grouped, and contextual factors were removed yielding a preliminary total of 36 domains (Table 1) which included domains such as disease activity, cognitive function, sleep, fatigue, anxiety, depression, stress, participation, pain interference, intimacy, and more.
Conclusion: Many candidate domains have been identified through the scoping literature review and focus group interviews. Further work is in process to finalize the list of the candidate domains to continue with a 4 round Delphi consensus voting exercise to achieve consensus on the new SLE CDS. The future goal for the SLE OMERACT Working Group is the instruments selection for each domain of the new CDS and appraisal of instruments measurement properties.
To cite this abstract in AMA style:
Nielsen W, Strand V, Simon L, Desai M, Parodis I, Kim A, Wallace D, Chaichian Y, Navarra S, Aranow C, MacKay M, Trotter K, Tayer-Shifman O, Duarte-Garcia A, Tam L, Ugarte-Gil M, Pons-Estel G, Reynolds J, Nikpour M, Papachristos D, Hoi A, Hendry A, Romero-Diaz J, Ramsey-Goldman R, Alrayes H, Almaghlouth I, Thayaparan A, Munoz-Grajales C, Howe A, Nowrouzi-Kia B, Anderson M, Bonilla D, Thumboo J, Mosca M, Aringer M, Johnson S, Drucker A, Morand E, Bruce I, Touma Z. Scoping Literature Review and Focus Groups Interviews to Identify Candidate Domains for the SLE OMERACT Core Domain Set [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/scoping-literature-review-and-focus-groups-interviews-to-identify-candidate-domains-for-the-sle-omeract-core-domain-set/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/scoping-literature-review-and-focus-groups-interviews-to-identify-candidate-domains-for-the-sle-omeract-core-domain-set/