Session Information
Date: Monday, November 14, 2022
Title: Systemic Sclerosis and Related Disorders – Clinical Poster III
Session Type: Poster Session D
Session Time: 1:00PM-3:00PM
Background/Purpose: Immune checkpoint inhibitors (ICIs) have proven to be efficacious in the treatment of multiple malignancies.[1] they are associated immunologic activation has been implicated in multiple immune-related adverse events (ir-AE) that can affect any organ system. Cutaneous manifestations, including pruritus, dermatitis, and burning, are some of the most common ir-AEs. At this time, there are very few reported cases of sclerodermoid skin changes in the setting of ICI therapy [2].
Methods: A retrospective chart review was conducted of patients seen at MD Anderson Cancer Center rheumatology clinic with suspected sclerodermoid ir-AEs following ICI therapy. Visit notes, blood work, imaging, and pathology records were evaluated to document patients’ disease course, therapy, and response to treatment. A literature search of similar published cases was also conducted.
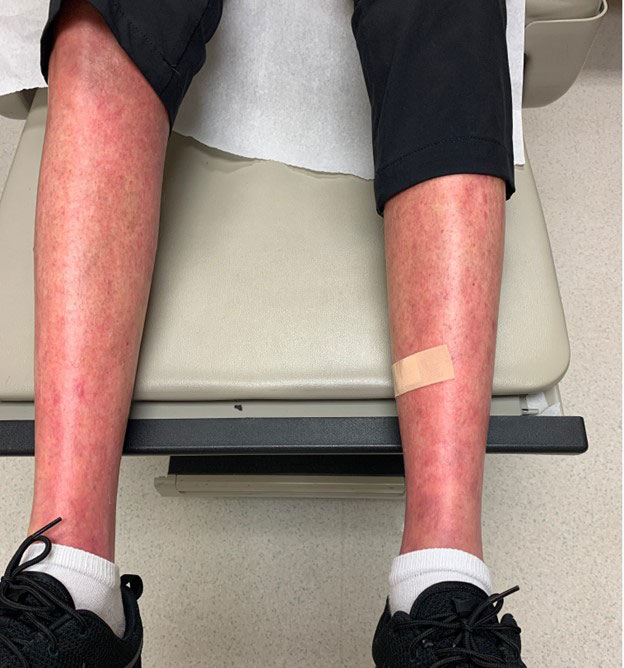
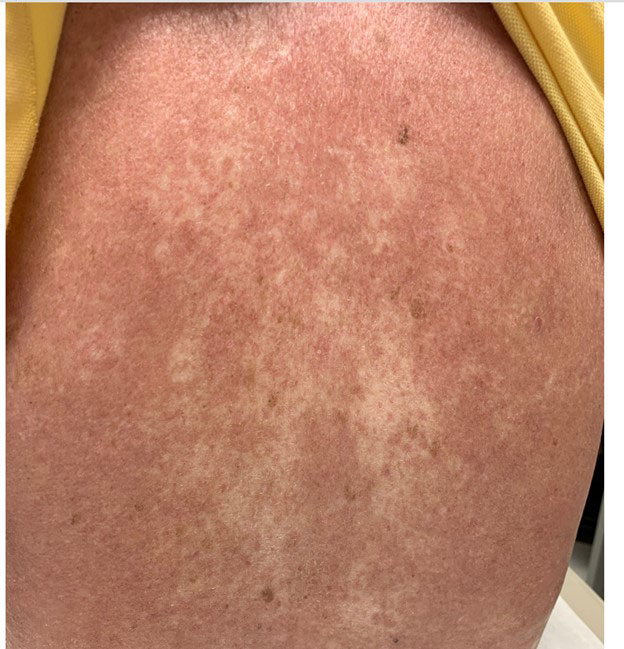
Results: This case series reports 3 ir-AEs manifesting as sclerodermoid skin changes. The first case describes a patient with no history of autoimmune disease that developed new skin thickening proximal to the bilateral knees and elbows 3 months after the initiation of nivolumab for malignant melanoma. Their symptoms became severely limiting and persisted after discontinuation of immunotherapy. autoimmune panel showed positive ANA 1:320, speckled pattern with negative full autoimmune and systemic sclerosis subsets. treated with high dose oral steroid tapers, mycophenolate, and tocilizumab. The second case also describes a patient with no known autoimmune history that developed bilateral skin thickening of the forearms and upper arms 1 month after initiation of pembrolizumab for mesothelioma that improved with discontinuation, high-dose topical steroids, and a course of low-dose systemic steroids. The final case details a significant worsening of previously well-controlled diffuse systemic sclerosis and development of new pyoderma gangrenosum 1 month after initiation of pembrolizumab for the treatment of metastatic renal cell carcinoma. The patient’s disease stabilized with discontinuation of pembrolizumab, initiation of hydroxychloroquine, and low dose systemic corticosteroids
Conclusion: Sclerodermoid cutaneous manifestations are rare ir-AEs that require prompt recognition in the setting of ICI therapy. These cases add to the expanding body of literature on this topic. Topical and/or systemic steroids for mild/moderate symptoms and discontinuation of the offending agent in severe cases per established cutaneous ir-AEs management guidelines lead to improvement in each case. Further research is needed to predict the risk for sclerodermoid ir-AEs, manage their symptoms, and weigh the risks and benefits of discontinuing ICI therapy in the setting of active malignancy.
1. Thallinger C, Fureder T, Preusser M, et al. Review of cancer treatment with immune checkpoint inhibitors: current concepts, expectations, limitations and pitfalls. Wien Klin Wochenschr. 2018;130(3–4):85-91.
2. Quach HT, Johnson DB, LeBoeuf NR, Zwerner JP, Dewan AK. Cutaneous adverse events caused by immune checkpoint inhibitors. J Am Acad Dermatol. 2021 Oct;85(4):956-966. doi: 10.1016/j.jaad.2020.09.054. Epub 2021 Jul 28. PMID: 34332798.
To cite this abstract in AMA style:
Chernis J, Buni M. Sclerodermoid Reaction Following Immune Checkpoint Inhibitor Therapy: A Case Series from MD Anderson Cancer Center [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/sclerodermoid-reaction-following-immune-checkpoint-inhibitor-therapy-a-case-series-from-md-anderson-cancer-center/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/sclerodermoid-reaction-following-immune-checkpoint-inhibitor-therapy-a-case-series-from-md-anderson-cancer-center/