Session Information
Date: Sunday, November 17, 2024
Title: Systemic Sclerosis & Related Disorders – Basic Science Poster I
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Interstitial lung disease (ILD) is present in a majority of systemic sclerosis (SSc) patients and the leading cause of SSc-related mortality. A subset of SSc-ILD patients share clinical characteristics with idiopathic pulmonary fibrosis (IPF), the most common form of ILD, including a usual interstitial pneumonia (UIP) pattern, male-sex, older age and poor prognosis. IPF is a disease of lung epithelial dysfunction and aberrant remodeling, however it is unclear whether these mechanisms are implicated in SSc-ILD. Our objective is to better understand SSc-ILD and if there are shared features of lung epithelial biology with IPF.
Methods: We performed single nuclei RNA sequencing (snRNAseq) of frozen explanted lung tissue of 30 individuals including 12 SSc (6 NSIP, 6 UIP), 6 IPF, and 12 controls. Data were processed using standardized computational pipelines. After subsetting epithelial cells, analysis comparing conditions was performed including cell proportion, differential gene expression, pseudo-time trajectory, and cell communication analysis using Kruskall Wallis, EdgeR, Monocle, and CellChat.
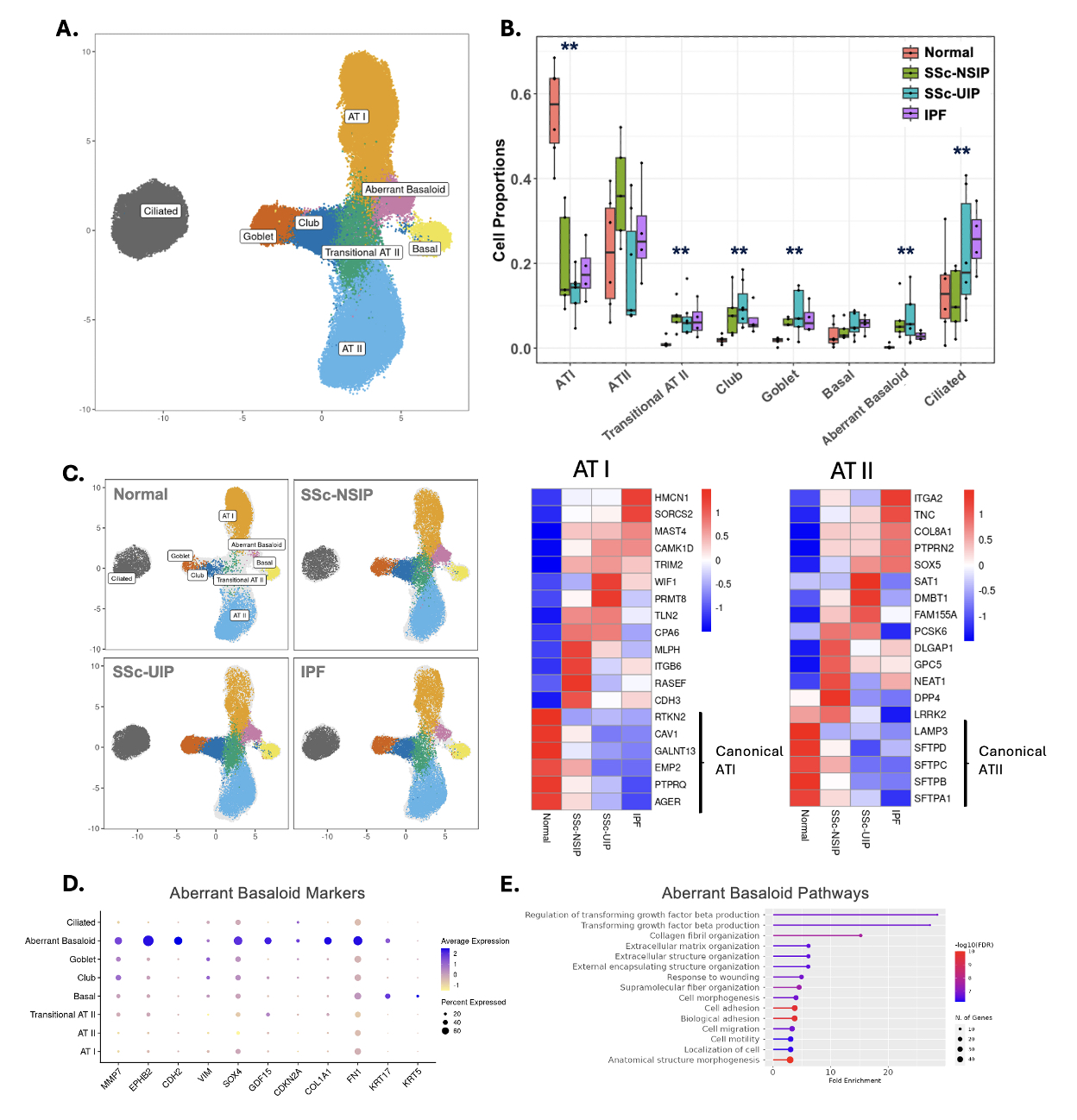
Results: A total of 72,128 lung epithelial cells were used in downstream analysis. Clustering revealed eight distinct clusters, including two pathologic cell types, transitional ATII and aberrant basaloid (Fig 1A-B). We found SSc-ILD and IPF were both characterized by profound loss of ATI cells, with reciprocal increase in several airway epithelial cell types including club, goblet, transitional ATII, and aberrant basaloid (p < 0.01). Ciliated cells were increased in SSc-UIP and IPF. Despite similar proportions of AT II cells among conditions, gene expression of ATII cells from SSc-ILD and IPF demonstrated a loss of canonical gene markers (i.e. SFTPC, SFTPA1) and increase in pathologic gene markers (i.e. DPP4, SOX5, COL8A1, ITGA2). This pattern was also present in ATI cells and most prominent in SSc-UIP and IPF (Fig 1C). A robust population of transitional ATII and aberrant basaloid cells, which have been reported in IPF but yet to be characterized in SSc-ILD, were identified across fibrotic diseases in our dataset. The transitional ATII cells expressed markers of both ATI and ATII cells although at lower levels. Aberrant basaloid cells in contrast were transcriptionally distinct and express markers of EMT (CDH2, COL1A1), cellular senescence (GDF15, CDKN2A), and previously defined IPF markers (MMP7, EPHB2). Pathway analysis revealed profibrotic pathways including TGFbeta production, extracellular matrix organization and wound healing (Fig 1D-E). Trajectory analysis suggests aberrant basaloid cells are derived from transitional ATII cells, which themselves arise from ATII cells, representing an aberrant deviation from ATII to ATI differentiation.
Conclusion: At the single cell level, most lung remodeling elements within the epithelial compartment were shared between IPF and SSc-ILD, with greater similarity between IPF and SSc-UIP. There was a proportional loss of alveolar epithelial cells with reciprocal expansion of airway epithelium and emergence of two pathologic cell types, yet to be characterized in SSc-ILD, involved in aberrant ATII differentiation and epithelial mesenchymal transition.
To cite this abstract in AMA style:
Yang M, Deiter F, Flynn E, Lee S, Neely J, Greenland J, Sirota M, Wolters P. Scleroderma Associated Interstitial Lung Disease Is Characterized by Aberrant Lung Epithelial Remodeling [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/scleroderma-associated-interstitial-lung-disease-is-characterized-by-aberrant-lung-epithelial-remodeling/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/scleroderma-associated-interstitial-lung-disease-is-characterized-by-aberrant-lung-epithelial-remodeling/